Consider the lattice energies of the following Group 2A compounds: BeH2, 3205 kJ/mol; MgH2, 2791 kJ/mol; CaH2, 2410 kJ/mol; SrH2, 2250 kJ/mol; BaH2, 2121 kJ/mol. (d) The lattice energy of ZnH2 is 2870 kJ/mol. Considering the trend in lattice enthalpies in the Group 2 compounds, predict which Group 2 element is most similar in ionic radius to the Zn2+ ion.
Ch.8 - Basic Concepts of Chemical Bonding

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 8, Problem 83
Construct a Born–Haber cycle for the formation of the hypothetical compound NaCl2, where the sodium ion has a 2+ charge (the second ionization energy for sodium is given in Table 7.2). (b) If we were to estimate the lattice energy of NaCl2 to be roughly equal to that of MgCl2 (2326 kJ/mol from Table 8.1), what value would you obtain for the standard enthalpy of formation, _x001F_Hf°, of NaCl2?
 Verified step by step guidance
Verified step by step guidance1
Identify the steps involved in the Born–Haber cycle for the formation of NaCl2. The cycle includes sublimation of sodium, ionization of sodium to Na2+, dissociation of Cl2, electron affinity of chlorine, and lattice energy of NaCl2.
Write the sublimation reaction for sodium: Na(s) → Na(g). Use the sublimation energy from a relevant table or source.
Consider the ionization of sodium to Na2+: Na(g) → Na+(g) + e− followed by Na+(g) → Na2+(g) + e−. Use the first and second ionization energies of sodium.
Write the dissociation reaction for chlorine: Cl2(g) → 2Cl(g). Use the bond dissociation energy for Cl2.
Consider the electron affinity of chlorine: Cl(g) + e− → Cl−(g). Use the electron affinity value for chlorine.
Estimate the lattice energy of NaCl2 using the given value for MgCl2 (2326 kJ/mol).
Apply Hess's Law to sum all the enthalpy changes: ΔHf° = ΔHsub + IE1 + IE2 + 1/2 D(Cl2) + EA(Cl) + U(NaCl2), where ΔHsub is the sublimation energy, IE1 and IE2 are the first and second ionization energies, D(Cl2) is the dissociation energy, EA(Cl) is the electron affinity, and U(NaCl2) is the lattice energy.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Born-Haber Cycle
The Born-Haber cycle is a thermodynamic cycle that relates the lattice energy of an ionic compound to its formation enthalpy. It involves several steps, including the sublimation of the solid, ionization of the metal, and the formation of ions from gaseous atoms. This cycle allows for the calculation of lattice energy by using Hess's law, which states that the total enthalpy change in a reaction is the sum of the enthalpy changes for each step.
Recommended video:
Guided course
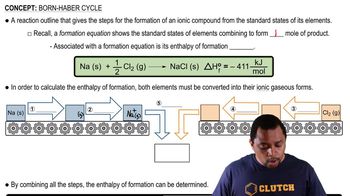
Born Haber Cycle
Lattice Energy
Lattice energy is the energy released when gaseous ions combine to form an ionic solid. It is a measure of the strength of the ionic bonds in the compound. Higher lattice energy indicates stronger interactions between ions, which typically results in higher melting points and greater stability of the ionic compound. In this context, estimating the lattice energy of NaCl2 based on the known value for MgCl2 provides a comparative basis for calculating the enthalpy of formation.
Recommended video:
Guided course

Lattice Energy
Enthalpy of Formation (_x001F_Hf°)
The standard enthalpy of formation (_x001F_Hf°) is the change in enthalpy when one mole of a compound is formed from its elements in their standard states. It is a crucial value in thermodynamics and helps predict the stability and reactivity of compounds. By using the Born-Haber cycle and the estimated lattice energy, one can calculate the _x001F_Hf° for NaCl2, providing insight into its thermodynamic properties.
Recommended video:
Guided course

Enthalpy of Formation
Related Practice
Textbook Question
Textbook Question
The ionic compound CaO crystallizes with the same structure as sodium chloride (Figure 8.3). (a) In this structure, how many O2- are in contact with each Ca2+ ion (Hint: Remember the pattern of ions shown in Figure 8.3 repeats over and over again in all three directions.)
Textbook Question
Construct a Born–Haber cycle for the formation of the hypothetical compound NaCl2, where the sodium ion has a 2+ charge (the second ionization energy for sodium is given in Table 7.2). (a) How large would the lattice energy need to be for the formation of NaCl2 to be exothermic?
Textbook Question
Consider the collection of nonmetallic elements O, P, Te, I, and B. (a) Which two would form the most polar single bond?
