Consider the lattice energies of the following Group 2A compounds: BeH2, 3205 kJ/mol; MgH2, 2791 kJ/mol; CaH2, 2410 kJ/mol; SrH2, 2250 kJ/mol; BaH2, 2121 kJ/mol. (a) What is the oxidation number of H in these compounds?
Ch.8 - Basic Concepts of Chemical Bonding

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 8, Problem 79d
Consider the lattice energies of the following Group 2A compounds: BeH2, 3205 kJ/mol; MgH2, 2791 kJ/mol; CaH2, 2410 kJ/mol; SrH2, 2250 kJ/mol; BaH2, 2121 kJ/mol. (d) The lattice energy of ZnH2 is 2870 kJ/mol. Considering the trend in lattice enthalpies in the Group 2 compounds, predict which Group 2 element is most similar in ionic radius to the Zn2+ ion.
 Verified step by step guidance
Verified step by step guidance1
Understand the concept of lattice energy: Lattice energy is the energy required to separate one mole of a solid ionic compound into gaseous ions. It is influenced by the charge and size of the ions involved.
Recognize the trend in lattice energies for Group 2A compounds: As you move down the group from BeH2 to BaH2, the lattice energy decreases. This is due to the increase in ionic radius, which results in weaker electrostatic forces between the ions.
Compare the lattice energy of ZnH2 with Group 2A compounds: ZnH2 has a lattice energy of 2870 kJ/mol, which is closest to MgH2 with a lattice energy of 2791 kJ/mol.
Relate lattice energy to ionic radius: Since lattice energy is inversely related to ionic radius, the element in Group 2A with a lattice energy closest to ZnH2 is likely to have an ionic radius similar to Zn2+.
Predict the most similar Group 2 element: Based on the comparison, Mg2+ is the Group 2 element most similar in ionic radius to Zn2+, as their lattice energies are closest.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Lattice Energy
Lattice energy is the amount of energy released when gaseous ions combine to form an ionic solid. It is a measure of the strength of the forces between the ions in an ionic compound. Higher lattice energy indicates stronger ionic bonds, which typically correlates with smaller ionic radii and higher charges on the ions.
Recommended video:
Guided course

Lattice Energy
Ionic Radius
Ionic radius refers to the size of an ion in a crystal lattice. Cations (positively charged ions) are generally smaller than their neutral atoms due to the loss of electrons, which reduces electron-electron repulsion. Understanding ionic radii is crucial for predicting the behavior of ions in compounds and their relative sizes, especially when comparing different elements.
Recommended video:
Guided course
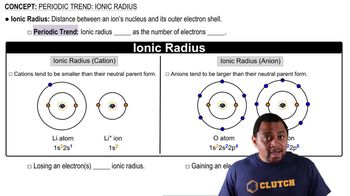
Ionic Radius
Trends in Group 2 Elements
In Group 2 elements, ionic radius increases down the group due to the addition of electron shells. This trend affects the lattice energies of their compounds, as larger ions typically lead to lower lattice energies. Recognizing these trends helps in predicting the properties of compounds formed by these elements, including their similarity to other ions, such as Zn<sup>2+</sup>.
Recommended video:
Guided course
Main Group Elements: Periodic Trends Example
Related Practice
Textbook Question
Textbook Question
Consider the lattice energies of the following Group 2A compounds: BeH2, 3205 kJ/mol; MgH2, 2791 kJ/mol; CaH2, 2410 kJ/mol; SrH2, 2250 kJ/mol; BaH2, 2121 kJ/mol. (c) Consider BeH2. Does it require 3205 kJ of energy to break one mole of the solid into its ions, or does breaking up one mole of solid into its ions release 3205 kJ of energy?
Textbook Question
The ionic compound CaO crystallizes with the same structure as sodium chloride (Figure 8.3). (a) In this structure, how many O2- are in contact with each Ca2+ ion (Hint: Remember the pattern of ions shown in Figure 8.3 repeats over and over again in all three directions.)
