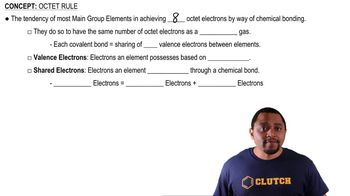
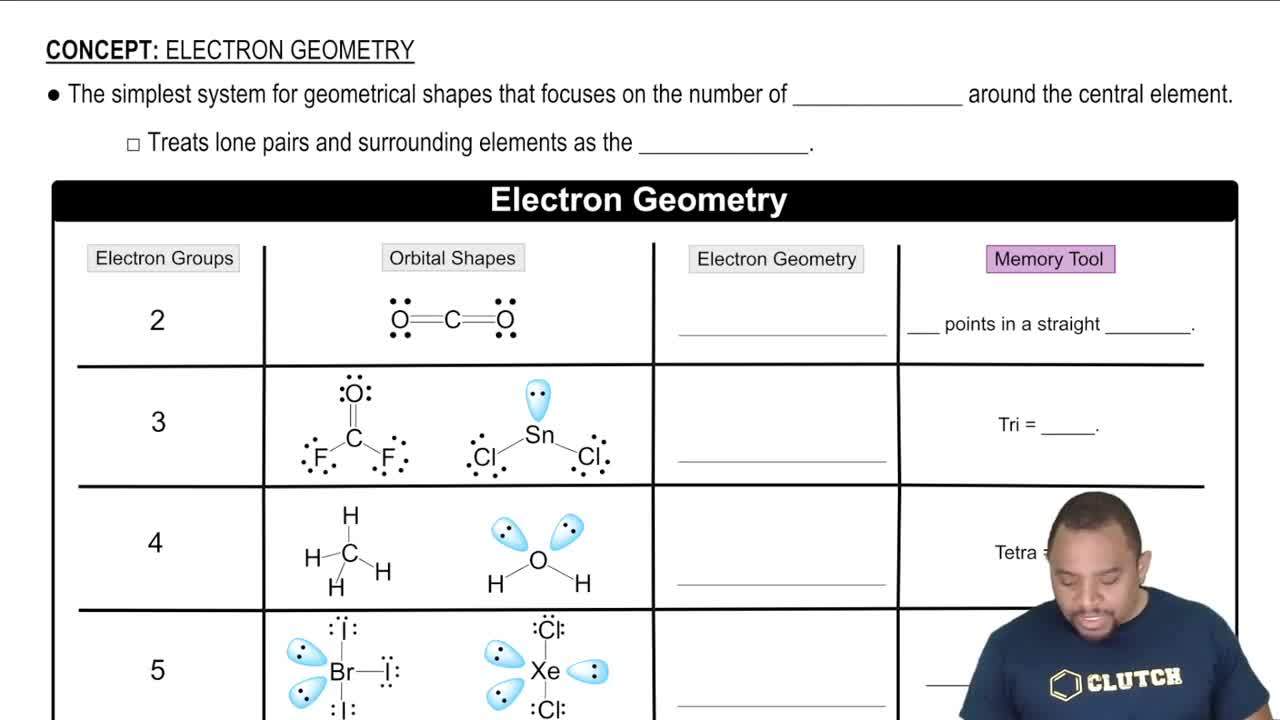
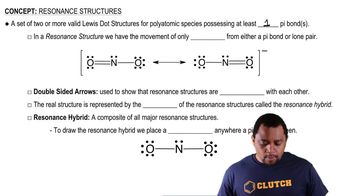
Mothballs are composed of naphthalene, C10H8, a molecule that consists of two six-membered rings of carbon fused along an edge, as shown in this incomplete Lewis structure:
(b) Do you expect the C—C bond lengths in the molecule to be similar to those of C—C single bonds, C ═C double bonds, or intermediate between C—C single and C ═C double bonds?