Textbook Question
For each of these Lewis symbols, indicate the group in the periodic table in which the element X belongs: (a)
3
views

 Verified step by step guidance
Verified step by step guidance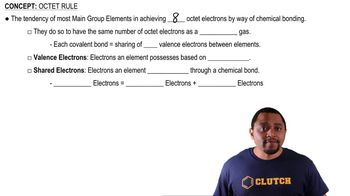


For each of these Lewis symbols, indicate the group in the periodic table in which the element X belongs: (a)
Illustrated are four ions — A, B, X, and Y— showing their relative ionic radii. The ions shown in red carry positive charges: a 2+ charge for A and a 1+ charge for B. Ions shown in blue carry negative charges: a 1- charge for X and a 2- charge for Y. (b) Among the combinations in part (a), which leads to the ionic compound having the largest lattice energy?
A portion of a two-dimensional 'slab' of NaCl(s) is shown here (see Figure 8.2) in which the ions are numbered. (a) Which colored balls must represent sodium ions?