Textbook Question
(c) Detailed calculations indicate that the effective nuclear charge is 5.6+ for the 3s electrons and 4.9+ for the 3p electrons. Why are the values for the 3s and 3p electrons different?

 Verified step by step guidance
Verified step by step guidance
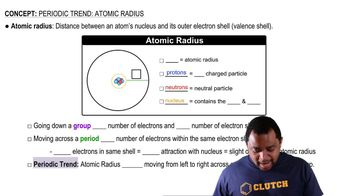

(c) Detailed calculations indicate that the effective nuclear charge is 5.6+ for the 3s electrons and 4.9+ for the 3p electrons. Why are the values for the 3s and 3p electrons different?
(d) If you remove a single electron from a P atom, which orbital will it come from?
The As ¬ As bond length in elemental arsenic is 2.48 Å. The Cl ¬ Cl bond length in Cl2 is 1.99 Å. (a) Based on these data, what is the predicted As ¬ Cl bond length in arsenic trichlo- ride, AsCl3, in which each of the three Cl atoms is bonded to the As atom?
The As ¬ As bond length in elemental arsenic is 2.48 Å. The Cl ¬ Cl bond length in Cl2 is 1.99 Å. (b) What bond length is predicted for AsCl3, using the atomic radii in Figure 7.7?