(b) Repeat these calculations using Slater’s rules.
Ch.7 - Periodic Properties of the Elements

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 7, Problem 82
As we move across a period of the periodic table, why do the sizes of the transition elements change more gradually than those of the representative elements?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the difference between transition elements and representative elements. Transition elements are found in the d-block of the periodic table, while representative elements are in the s-block and p-block.
Step 2: Recognize that atomic size is influenced by the effective nuclear charge, which is the net positive charge experienced by an electron in an atom. This is affected by the number of protons in the nucleus and the shielding effect of inner electrons.
Step 3: Note that as you move across a period, the number of protons increases, which generally increases the effective nuclear charge, pulling electrons closer to the nucleus and reducing atomic size.
Step 4: In transition elements, the additional electrons are added to the inner d-subshell, which provides some shielding and reduces the increase in effective nuclear charge compared to representative elements where electrons are added to the outermost shell.
Step 5: Conclude that the gradual change in size of transition elements is due to the balance between increased nuclear charge and the shielding effect of the d-electrons, leading to a less pronounced decrease in atomic size compared to representative elements.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Periodic Trends
Periodic trends refer to the predictable patterns observed in the properties of elements as one moves across a period or down a group in the periodic table. These trends include atomic size, ionization energy, and electronegativity. Understanding these trends helps explain why certain elements exhibit gradual changes in size compared to others.
Recommended video:
Guided course
Periodic Trends
Transition Elements
Transition elements, found in the d-block of the periodic table, have partially filled d-orbitals. This unique electron configuration leads to more complex interactions between electrons, resulting in smaller and more gradual changes in atomic size across a period compared to representative elements, which have more straightforward electron configurations.
Recommended video:
Guided course
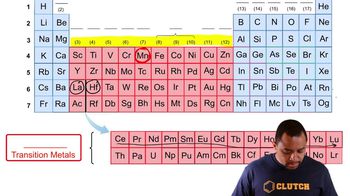
Transition Metals and Representative Elements
Effective Nuclear Charge
Effective nuclear charge (Z_eff) is the net positive charge experienced by an electron in a multi-electron atom. As one moves across a period, Z_eff increases due to the addition of protons in the nucleus while shielding remains relatively constant. This increase in Z_eff contributes to the gradual decrease in atomic size for transition elements, as the increased nuclear attraction pulls electrons closer to the nucleus.
Recommended video:
Guided course
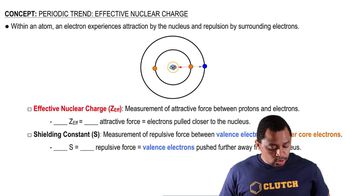
Effective Nuclear Charge
Related Practice
Textbook Question
Textbook Question
(c) Detailed calculations indicate that the effective nuclear charge is 5.6+ for the 3s electrons and 4.9+ for the 3p electrons. Why are the values for the 3s and 3p electrons different?
Textbook Question
(d) If you remove a single electron from a P atom, which orbital will it come from?
Textbook Question
In Table 7.8, the bonding atomic radius of neon is listed as58 pm, whereas that for xenon is listed as 140 pm. A classmateof yours states that the value for Xe is more realisticthan the one for Ne. Is she correct? If so, what is the basisfor her statement?
Textbook Question
The As ¬ As bond length in elemental arsenic is 2.48 Å. The Cl ¬ Cl bond length in Cl2 is 1.99 Å. (a) Based on these data, what is the predicted As ¬ Cl bond length in arsenic trichlo- ride, AsCl3, in which each of the three Cl atoms is bonded to the As atom?
Textbook Question
The As ¬ As bond length in elemental arsenic is 2.48 Å. The Cl ¬ Cl bond length in Cl2 is 1.99 Å. (b) What bond length is predicted for AsCl3, using the atomic radii in Figure 7.7?
