The first ionization energy of the oxygen molecule is the energy required for the following process: O21g2¡O2 +1g2 + e- The energy needed for this process is 1175 kJ>mol, very similar to the first ionization energy of Xe. Would you expect O2 to react with F2? If so, suggest a product or products of this reaction.

Elemental barium reacts more violently with water than does elemental calcium. Which of the following best explains this difference in reactivity? (i) Calcium has greater metallic character than does barium. (ii) The electron affinity of calcium is smaller than that of barium. (iii) The first and second ionization energies of barium are less than those of calcium. (iv) The atomic radius of barium is smaller than that of calcium. (v) The ionic radius of the barium ion is larger than that of the calcium ion.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Metallic Character
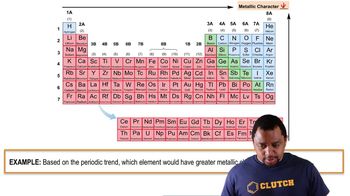
Ionization Energy

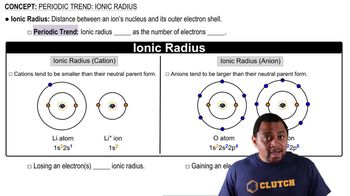
Atomic and Ionic Radius
It is possible to define metallic character as we do in this book and base it on the reactivity of the element and the ease with which it loses electrons. Alternatively, one could measure how well electricity is conducted by each of the elements to determine how 'metallic' the elements are. On the basis of conductivity, there is not much of a trend in the periodic table: Silver is the most conductive metal, and manganese the least. Look up the first ionization energies of silver and manganese; which of these two elements would you call more metallic based on the way we define it in this book?
Which of the following is the expected product of the reaction of K(s) and H2(g)? (i) KH(s), (ii) K2H(s), (iii) KH2(s), (iv) K2H2(s), or (v) K(s) and H2(g) will not react with one another.
A historian discovers a nineteenth-century notebook in which some observations, dated 1822, were recorded on a substance thought to be a new element. Here are some of the data recorded in the notebook: 'Ductile, silver-white, metallic looking. Softer than lead. Unaffected by water. Stable in air. Melting point: 153 °C. Density: 7.3 g>cm3. Electrical conductivity: 20% that of copper. Hardness: About 1% as hard as iron. When 4.20 g of the unknown is heated in an excess of oxygen, 5.08 g of a white solid is formed. The solid could be sublimed by heating to over 800 °C.' (a) Using information in the text and the CRC Handbook of Chemistry and Physics, and making allowances for possible variations in numbers from current values, identify the element reported.
