
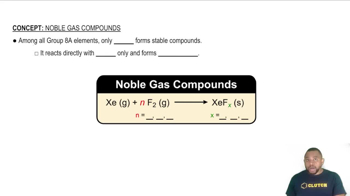
(e) While it is possible to form BiF5 in the manner just described, pentahalides of bismuth are not known for the other halogens. Explain why the pentahalide might form with fluorine but not with the other halogens. How does the behavior of bismuth relate to the fact that xenon reacts with fluorine to form compounds but not with the other halogens?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Halogen Reactivity and Size
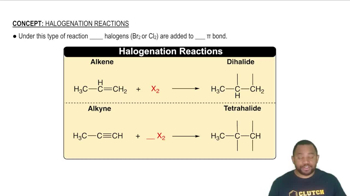
Oxidation States and Stability

Noble Gas Chemistry
When magnesium metal is burned in air (Figure 3.6), two products are produced. One is magnesium oxide, MgO. The other is the product of the reaction of Mg with molecular nitrogen, magnesium nitride. When water is added to magnesium nitride, it reacts to form magnesium oxide and ammonia gas. (c) In an experiment, a piece of magnesium ribbon is burned in air in a crucible. The mass of the mixture of MgO and magnesium nitride after burning is 0.470 g. Water is added to the crucible, further reaction occurs, and the crucible is heated to dryness until the final product is 0.486 g of MgO. What was the mass percentage of magnesium nitride in the mixture obtained after the initial burning?
When magnesium metal is burned in air (Figure 3.6), two products are produced. One is magnesium oxide, MgO. The other is the product of the reaction of Mg with molecular nitrogen, magnesium nitride. When water is added to magnesium nitride, it reacts to form magnesium oxide and ammonia gas. (d) Magnesium nitride can also be formed by reaction of the metal with ammonia at high temperature. Write a balanced equation for this reaction. If a 6.3-g Mg ribbon reacts with 2.57 g NH31g2 and the reaction goes to completion, which component is the limiting reactant? What mass of H21g2 is formed in the reaction?
Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) because KO2 reacts with CO2 to release molecular oxygen. Experiments indicate that 2 mol of KO2(s) react with each mole of CO2(g). (a) The products of the reaction are K2CO3(s) and O2(g). Write a balanced equation for the reaction between KO2(s) and CO2(g).
Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) because KO2 reacts with CO2 to release molecular oxygen. Experiments indicate that 2 mol of KO2(s) react with each mole of CO2(g). (c) What mass of KO2(s) is needed to consume 18.0 g CO2(g)? What mass of O2(g) is produced during this reaction?
