When magnesium metal is burned in air (Figure 3.6), two products are produced. One is magnesium oxide, MgO. The other is the product of the reaction of Mg with molecular nitrogen, magnesium nitride. When water is added to magnesium nitride, it reacts to form magnesium oxide and ammonia gas. (d) Magnesium nitride can also be formed by reaction of the metal with ammonia at high temperature. Write a balanced equation for this reaction. If a 6.3-g Mg ribbon reacts with 2.57 g NH31g2 and the reaction goes to completion, which component is the limiting reactant? What mass of H21g2 is formed in the reaction?
Ch.7 - Periodic Properties of the Elements

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 7, Problem 115c
Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) because KO2 reacts with CO2 to release molecular oxygen. Experiments indicate that 2 mol of KO2(s) react with each mole of CO2(g). (c) What mass of KO2(s) is needed to consume 18.0 g CO2(g)? What mass of O2(g) is produced during this reaction?
 Verified step by step guidance
Verified step by step guidance1

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between the reactants and products in a chemical reaction. It allows us to calculate the amounts of substances consumed and produced based on balanced chemical equations. In this case, knowing the stoichiometric ratio between KO2 and CO2 is essential to determine how much KO2 is needed to react with a given mass of CO2.
Recommended video:
Guided course

Stoichiometry Concept
Molar Mass
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). It is crucial for converting between the mass of a substance and the number of moles. To solve the problem, we need to calculate the molar mass of KO2 and CO2 to find out how many grams of KO2 are required to react with 18.0 g of CO2.
Recommended video:
Guided course

Molar Mass Concept
Gas Laws
Gas laws describe the behavior of gases in relation to pressure, volume, temperature, and the number of moles. In this context, understanding how the reaction produces O2 gas is important, as it relates to the ideal gas law and the conditions under which gases are measured. The reaction's stoichiometry will also help determine the amount of O2 produced from the reaction of KO2 with CO2.
Recommended video:
Guided course
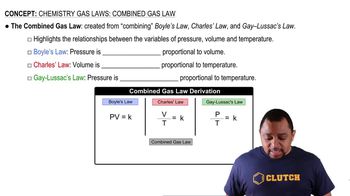
Combined Gas Law
Related Practice
Textbook Question
Textbook Question
Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) because KO2 reacts with CO2 to release molecular oxygen. Experiments indicate that 2 mol of KO2(s) react with each mole of CO2(g). (a) The products of the reaction are K2CO3(s) and O2(g). Write a balanced equation for the reaction between KO2(s) and CO2(g).
