Textbook Question
Neutron diffraction is an important technique for determining the structures of molecules. Calculate the velocity of a neutron needed to achieve a wavelength of 125 pm. (Refer to the inside cover for the mass of the neutron.)

 Verified step by step guidance
Verified step by step guidance


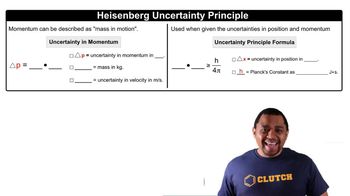
Using Heisenberg's uncertainty principle, calculate the uncertainty in the position of (a) a 1.50-mg mosquito moving at a speed of 1.40 m/s if the speed is known to within {0.01 m/s;
Using Heisenberg's uncertainty principle, calculate the uncertainty in the position of (b) a proton moving at a speed of 15.00 { 0.012 * 104 m/s. (The mass of a proton is given in the table of fundamental constants in the inside cover of the text.)
(a) For n = 4, what are the possible values of l?
How many unique combinations of the quantum numbers l and ml are there when (a) n = 1 (b) n = 5?
Give the numerical values of n and l corresponding to each of the following orbital designations: (a) 3p (b) 2s (c) 4f