A sodium ion, Na+, with a charge of 1.6⨉10-19 C and a chloride ion, Cl - , with charge of -1.6⨉10-19 C, are separated by a distance of 0.50 nm. How much work would be required to increase the separation of the two ions to an infinite distance?

(a) Which of the following cannot leave or enter a closed system: heat, work, or matter? (b) Which cannot leave or enter an isolated system? (c) What do we call the part of the universe that is not part of the system?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
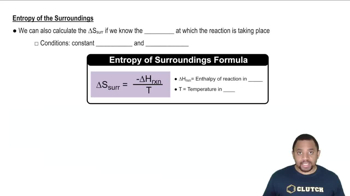
Closed System
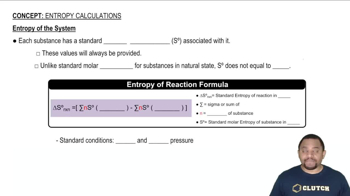
Isolated System

Surroundings
A magnesium ion, Mg2+, with a charge of 3.2⨉10-19 C and an oxide ion, O2-, with a charge of -3.2⨉10-19 C, are separated by a distance of 0.35 nm. How much work would be required to increase the separation of the two ions to an infinite distance?
In a thermodynamic study, a scientist focuses on the properties of a solution in an apparatus as illustrated. A solution is continuously flowing into the apparatus at the top and out at the bottom, such that the amount of solution in the apparatus is constant with time. (a) Is the solution in the apparatus a closed system, open system, or isolated system?
In a thermodynamic study, a scientist focuses on the properties of a solution in an apparatus as illustrated. A solution is continuously flowing into the apparatus at the top and out at the bottom, such that the amount of solution in the apparatus is constant with time. (b) If the inlet and outlet were closed, what type of system would it be
(a) According to the first law of thermodynamics, what quantity is conserved?
