Textbook Question
(b) What is the change in potential energy if the distance separating the two electrons is increased to 1.0 nm?

 Verified step by step guidance
Verified step by step guidance
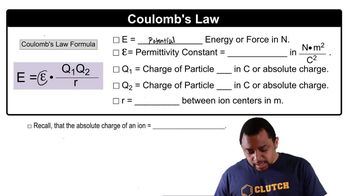


(b) What is the change in potential energy if the distance separating the two electrons is increased to 1.0 nm?
(c) Does the potential energy of the two particles increase or decrease when the distance is increased to 1.0 nm?
A sodium ion, Na+, with a charge of 1.6⨉10-19 C and a chloride ion, Cl - , with charge of -1.6⨉10-19 C, are separated by a distance of 0.50 nm. How much work would be required to increase the separation of the two ions to an infinite distance?
A magnesium ion, Mg2+, with a charge of 3.2⨉10-19 C and an oxide ion, O2-, with a charge of -3.2⨉10-19 C, are separated by a distance of 0.35 nm. How much work would be required to increase the separation of the two ions to an infinite distance?