Suppose you have 3.00 g of powdered zinc metal, 3.00g of powdered silver metal and 500.0 mL of a 0.2 M copper(II) nitrate solution. (d) What is the molarity of Cu2+ ions in the resulting solution?
Ch.4 - Reactions in Aqueous Solution

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 4, Problem 107
The discovery of hafnium, element number 72, provideda controversial episode in chemistry. G. Urbain, a Frenchchemist, claimed in 1911 to have isolated an elementnumber 72 from a sample of rare earth (elements 58–71)compounds. However, Niels Bohr believed that hafniumwas more likely to be found along with zirconium thanwith the rare earths. D. Coster and G. von Hevesy, workingin Bohr's laboratory in Copenhagen, showed in 1922 thatelement 72 was present in a sample of Norwegian zircon,an ore of zirconium. (The name hafnium comes from theLatin name for Copenhagen, Hafnia). (c) Solid zirconiumdioxide, ZrO2, reacts with chlorine gas in the presenceof carbon. The products of the reaction are ZrCl4 and twogases, CO2 and CO in the ratio 1:2. Write a balanced chemicalequation for the reaction.
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants and products in the reaction: ZrO_2 (solid), Cl_2 (gas), and C (solid) are the reactants, while ZrCl_4 (solid), CO_2 (gas), and CO (gas) are the products.
Write the unbalanced chemical equation: ZrO_2 + Cl_2 + C -> ZrCl_4 + CO_2 + CO.
Balance the zirconium (Zr) atoms: There is 1 Zr atom on both sides, so they are already balanced.
Balance the chlorine (Cl) atoms: There are 4 Cl atoms in ZrCl_4, so you need 2 Cl_2 molecules on the reactant side to provide 4 Cl atoms.
Balance the carbon (C) and oxygen (O) atoms: Use the given ratio of CO_2 to CO (1:2) to balance the carbon and oxygen atoms, ensuring the total number of each type of atom is the same on both sides of the equation.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations is a fundamental skill in chemistry that ensures the law of conservation of mass is upheld. This law states that matter cannot be created or destroyed in a chemical reaction, meaning the number of atoms of each element must be the same on both sides of the equation. To balance an equation, coefficients are adjusted in front of the chemical formulas to achieve equal numbers of each type of atom. This process is crucial for accurately representing the stoichiometry of a reaction.
Recommended video:
Guided course

Balancing Chemical Equations
Stoichiometry
Stoichiometry is the quantitative relationship between the reactants and products in a chemical reaction. It allows chemists to predict the amounts of substances consumed and produced in a reaction based on balanced equations. Understanding stoichiometry is essential for calculating yields, determining limiting reactants, and scaling reactions for practical applications. In the context of the given reaction, stoichiometry helps in understanding the molar ratios of reactants and products involved.
Recommended video:
Guided course

Stoichiometry Concept
Chemical Reaction Types
Chemical reactions can be classified into several types, including synthesis, decomposition, single replacement, and double replacement reactions. The reaction described in the question involves the synthesis of zirconium tetrachloride (ZrCl4) from zirconium dioxide (ZrO2) and chlorine gas (Cl2), with carbon acting as a reducing agent. Recognizing the type of reaction helps in predicting the products and understanding the mechanisms involved, which is vital for writing accurate balanced equations.
Recommended video:
Guided course
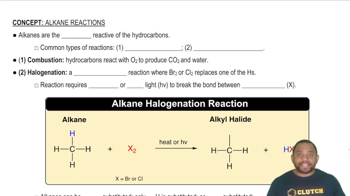
Common Types of Alkane Reactions
Related Practice
Textbook Question
Textbook Question
(a) By titration, 15.0 mL of 0.1008 M sodium hydroxide is needed to neutralize a 0.2053-g sample of a weak acid. What is the molar mass of the acid if it is monoprotic?
Textbook Question
(b) An elemental analysis of the acid indicates that it is composed of 5.89% H, 70.6% C, and 23.5% O by mass. What is its molecular formula?
Textbook Question
A sample of 8.69 g of Zn(OH)2 is added to 155.0 mL of 0.750 M H2SO4. (b) Which is the limiting reactant in the reaction?
Textbook Question
A sample of 8.69 g of Zn(OH)2 is added to 155.0 mL of 0.750 M H2SO4. (c) How many moles of Zn(OH)2, H2SO4, ZnSO4 are present after the reaction is complete?
