Determine the oxidation number for the indicated element in each of the following substances: (c) P in AgPF6
Ch.4 - Reactions in Aqueous Solution

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 4, Problem 49a,b
Determine the oxidation number for the indicated element in each of the following substances: (a) S in SO3 (b) Ti in TiCl4
 Verified step by step guidance
Verified step by step guidance1
Identify the oxidation number of oxygen, which is typically -2.
Let the oxidation number of sulfur (S) be x.
Write the chemical formula for sulfur trioxide: SO_3.
Set up the equation based on the sum of oxidation numbers in a neutral compound: x + 3(-2) = 0.
Solve for x to find the oxidation number of sulfur.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
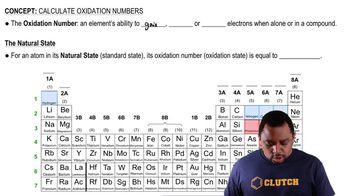
Oxidation Number
The oxidation number, or oxidation state, is a theoretical charge assigned to an atom in a compound based on the assumption that electrons are completely transferred. It helps in understanding the electron distribution in molecules and is crucial for identifying redox reactions. Oxidation numbers can be positive, negative, or zero, depending on the atom's electron gain or loss.
Recommended video:
Guided course
Oxidation Numbers
Rules for Assigning Oxidation Numbers
There are specific rules for assigning oxidation numbers, such as the fact that the oxidation number of an element in its elemental form is zero, and for monoatomic ions, it equals the charge of the ion. In compounds, hydrogen typically has an oxidation number of +1, oxygen usually has -2, and the sum of oxidation numbers in a neutral compound must equal zero. These rules guide the determination of oxidation states in various substances.
Recommended video:
Guided course

Oxidation Number Rules
Sulfur in SO3
In sulfur trioxide (SO3), sulfur is the central atom surrounded by three oxygen atoms. To find the oxidation number of sulfur, we apply the rules for oxidation states. Given that each oxygen has an oxidation number of -2, the total contribution from oxygen is -6. To balance this in a neutral molecule, sulfur must have an oxidation number of +6, indicating it has lost six electrons in this compound.
Recommended video:
Guided course
Hess's Law and Equilibrium Constant
Related Practice
Textbook Question
Textbook Question
Determine the oxidation number of sulfur in each of the following substances: (d) hydrogen sulfide, H2S
Textbook Question
Determine the oxidation number of sulfur in each of the following substances: (e) Locate sulfur in the periodic table in Exercise 4.47; what region is it in?
1
views
Textbook Question
Determine the oxidation number of sulfur in each of the following substances: (f) Which region(s) of the periodic table contains elements that can adopt both positive and negative oxidation numbers?
Textbook Question
Determine the oxidation number for the indicated element in each of the following substances: (d) N in HNO3
Textbook Question
Determine the oxidation number for the indicated element in each of the following substances: (e) Pt in PtCl4 (f) O in OF2.
