Ch.22 - Chemistry of the Nonmetals

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 22, Problem 22
What does hydrogen have in common with the halogens? Explain.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the periodic table placement of hydrogen and the halogens. Hydrogen is located in Group 1, while the halogens are in Group 17.
Step 2: Recognize that both hydrogen and the halogens have one electron less than a full outer shell. Hydrogen has one electron, needing one more to complete its shell, similar to halogens which have seven valence electrons and need one more to complete their octet.
Step 3: Note the chemical reactivity similarity. Both hydrogen and halogens tend to form bonds by gaining or sharing one electron to achieve a stable electron configuration.
Step 4: Consider the types of compounds they form. Hydrogen can form compounds like hydrogen halides (e.g., HCl, HF) with halogens, where it shares its electron to form a covalent bond.
Step 5: Reflect on the non-metallic nature of both. Hydrogen, like the halogens, is a non-metal and shares similar properties such as forming diatomic molecules (H2, Cl2, etc.) in their elemental state.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Group Properties in the Periodic Table
Elements in the periodic table are organized into groups based on similar chemical properties. Hydrogen, while not a halogen, shares some characteristics with the halogens (Group 17) due to its electron configuration. Both groups can form diatomic molecules and exhibit similar reactivity patterns, particularly in forming acids with nonmetals.
Recommended video:
Guided course
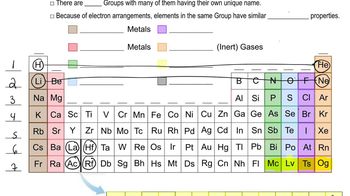
Periodic Table: Group Names
Electron Configuration
The electron configuration of an element describes the distribution of electrons in its atomic orbitals. Hydrogen has one electron in its outer shell, similar to halogens, which have seven valence electrons. This similarity allows hydrogen to participate in similar chemical reactions, such as forming bonds with other elements to achieve a stable electron configuration.
Recommended video:
Guided course

Electron Configuration Example
Acid-Base Chemistry
Hydrogen and halogens are involved in acid-base reactions, where hydrogen can act as a proton donor (acid) and halogens can form acids when combined with hydrogen. For example, hydrogen chloride (HCl) is a strong acid formed from hydrogen and chlorine. This relationship highlights the reactivity of hydrogen in forming compounds that exhibit acidic properties, akin to the behavior of halogens.
Recommended video:
Guided course

Arrhenius Acids and Bases
Related Practice
