Ch.22 - Chemistry of the Nonmetals

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 22, Problem 29
Complete the exercises below: Why does xenon form stable compounds with fluorine, whereas argon does not?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the electronic configuration of xenon and argon. Xenon has the electronic configuration [Kr] 4d^{10} 5s^{2} 5p^{6}, while argon has [Ne] 3s^{2} 3p^{6}.
Step 2: Recognize that xenon is a noble gas with a complete outer shell, but it has empty d orbitals in its valence shell that can be used for bonding.
Step 3: Note that argon, being a smaller noble gas, does not have available d orbitals in its valence shell, limiting its ability to expand its octet and form compounds.
Step 4: Consider the electronegativity and reactivity of fluorine, which is highly electronegative and can attract electrons from xenon, allowing xenon to form stable compounds like XeF_2, XeF_4, and XeF_6.
Step 5: Conclude that the ability of xenon to form stable compounds with fluorine is due to its larger atomic size and the availability of d orbitals, which argon lacks, preventing it from forming similar compounds.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Noble Gas Stability
Noble gases, such as argon and xenon, have a full valence shell, making them generally unreactive. However, xenon can form stable compounds due to its larger atomic size and the ability to expand its octet, allowing it to accommodate more than eight electrons. In contrast, argon, being smaller, does not have the same capacity to form stable compounds with highly electronegative elements like fluorine.
Recommended video:
Guided course
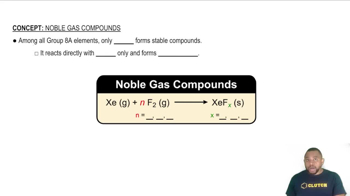
Noble Gas Compounds
Chemical Reactivity of Halogens
Halogens, such as fluorine, are highly reactive due to their strong tendency to gain an electron and achieve a stable octet configuration. When reacting with xenon, fluorine can form stable compounds like xenon difluoride (XeF2) because xenon can accommodate additional electrons. Argon, however, does not readily react with halogens, as it lacks the ability to form stable bonds with them.
Recommended video:
Guided course
Halogenation Reactions
Bonding and Molecular Orbital Theory
Molecular orbital theory explains how atomic orbitals combine to form molecular orbitals, which can be occupied by electrons. In xenon-fluorine compounds, the overlap of orbitals allows for the formation of stable bonds. Argon, lacking the ability to form such molecular orbitals with fluorine, does not participate in bonding, resulting in its inability to form stable compounds.
Recommended video:
Guided course

Molecular Orbital Theory
Related Practice
