Ch.22 - Chemistry of the Nonmetals

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 22, Problem 18
Complete the exercises below. Complete and balance the following equation: b. C₃H₇OH (l) + O₂ (g) →
 Verified step by step guidance
Verified step by step guidance1
Identify the type of chemical reaction. This is a combustion reaction where an alcohol reacts with oxygen to produce carbon dioxide and water.
Write the unbalanced chemical equation: \( \text{C}_3\text{H}_7\text{OH} (l) + \text{O}_2 (g) \rightarrow \text{CO}_2 (g) + \text{H}_2\text{O} (g) \).
Balance the carbon atoms first. There are 3 carbon atoms in \( \text{C}_3\text{H}_7\text{OH} \), so place a coefficient of 3 in front of \( \text{CO}_2 \).
Next, balance the hydrogen atoms. There are 8 hydrogen atoms in \( \text{C}_3\text{H}_7\text{OH} \), so place a coefficient of 4 in front of \( \text{H}_2\text{O} \).
Finally, balance the oxygen atoms. Count the total oxygen atoms needed on the product side and adjust the coefficient of \( \text{O}_2 \) accordingly.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations involves ensuring that the number of atoms of each element is the same on both sides of the equation. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. To balance an equation, coefficients are adjusted in front of the chemical formulas to achieve equal atom counts.
Recommended video:
Guided course

Balancing Chemical Equations
Combustion Reactions
Combustion reactions are a type of chemical reaction where a substance (usually a hydrocarbon) reacts with oxygen to produce carbon dioxide and water, releasing energy in the form of heat and light. In the case of the given equation, the alcohol C₃H₇OH is undergoing combustion, which requires balancing the reactants and products to reflect the complete reaction.
Recommended video:
Guided course
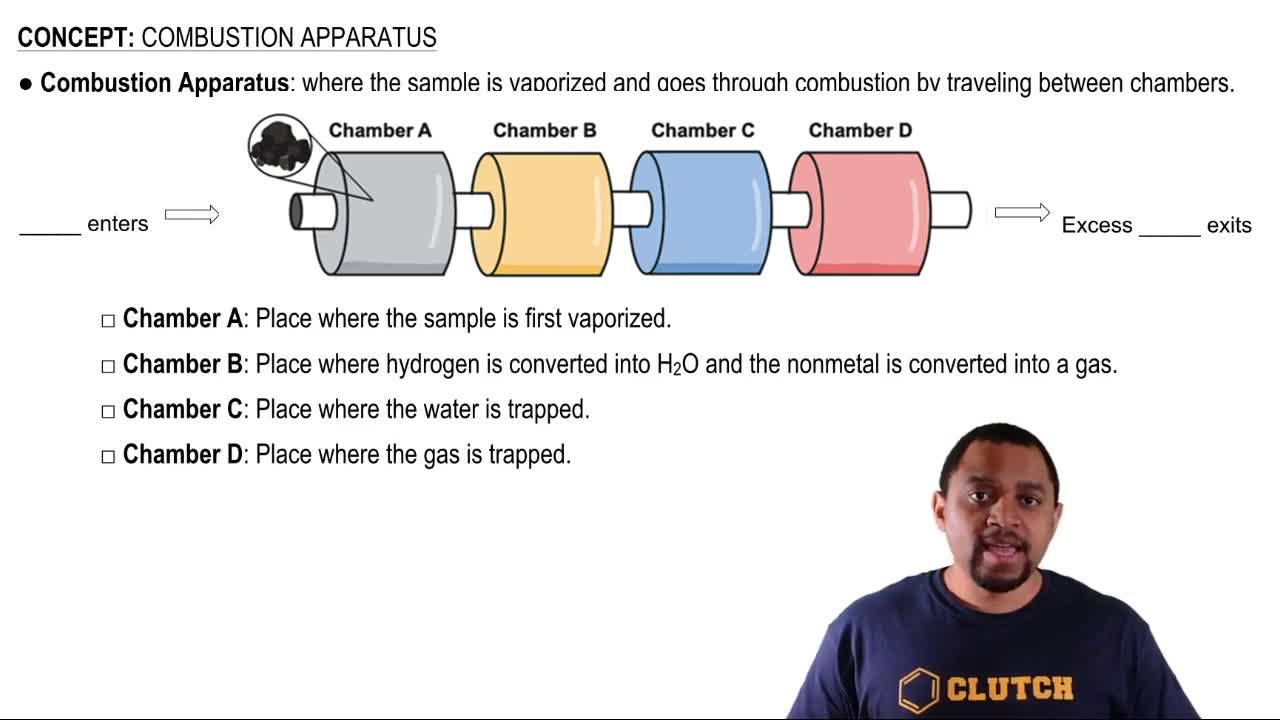
Combustion Apparatus
Stoichiometry
Stoichiometry is the calculation of reactants and products in chemical reactions based on the balanced equation. It allows chemists to predict the amounts of substances consumed and produced in a reaction. Understanding stoichiometry is essential for balancing equations, as it provides the ratios needed to ensure that all atoms are accounted for in the reaction.
Recommended video:
Guided course

Stoichiometry Concept
Related Practice
