Ch.22 - Chemistry of the Nonmetals

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 22, Problem 18
Complete the exercises below. Complete and balance the following equation: e. Na₂S (s) + HCl (aq) →
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants and products in the chemical equation. The reactants are sodium sulfide (Na₂S) and hydrochloric acid (HCl). The products will be determined by the reaction between these two compounds.
Predict the products of the reaction. Sodium sulfide (Na₂S) reacts with hydrochloric acid (HCl) to form hydrogen sulfide (H₂S) gas and sodium chloride (NaCl) in aqueous solution.
Write the unbalanced chemical equation using the predicted products: Na₂S (s) + HCl (aq) → H₂S (g) + NaCl (aq).
Balance the chemical equation by ensuring the same number of each type of atom on both sides of the equation. Start by balancing the sodium (Na) atoms, then the chlorine (Cl) atoms, and finally the hydrogen (H) and sulfur (S) atoms.
Verify that the equation is balanced by counting the atoms of each element on both sides of the equation. Adjust coefficients as necessary to achieve balance.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations is essential to ensure that the law of conservation of mass is upheld, meaning the number of atoms of each element must be the same on both sides of the equation. This involves adjusting coefficients in front of the chemical formulas to achieve balance without changing the actual compounds involved.
Recommended video:
Guided course

Balancing Chemical Equations
Types of Chemical Reactions
Understanding the types of chemical reactions, such as acid-base reactions, is crucial for predicting the products of a reaction. In this case, sodium sulfide (Na₂S) reacts with hydrochloric acid (HCl), which typically results in the formation of a salt and hydrogen sulfide gas (H₂S), illustrating an acid-base neutralization.
Recommended video:
Guided course
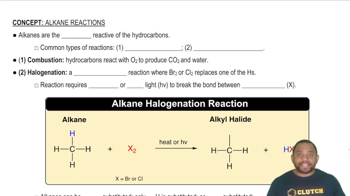
Common Types of Alkane Reactions
States of Matter in Reactions
Recognizing the states of matter (solid, liquid, gas, aqueous) is important for interpreting chemical equations. In the given reaction, Na₂S is a solid (s) and HCl is an aqueous solution (aq), which influences the reaction dynamics and the physical state of the products formed, such as whether a gas is released or a precipitate forms.
Recommended video:
Guided course
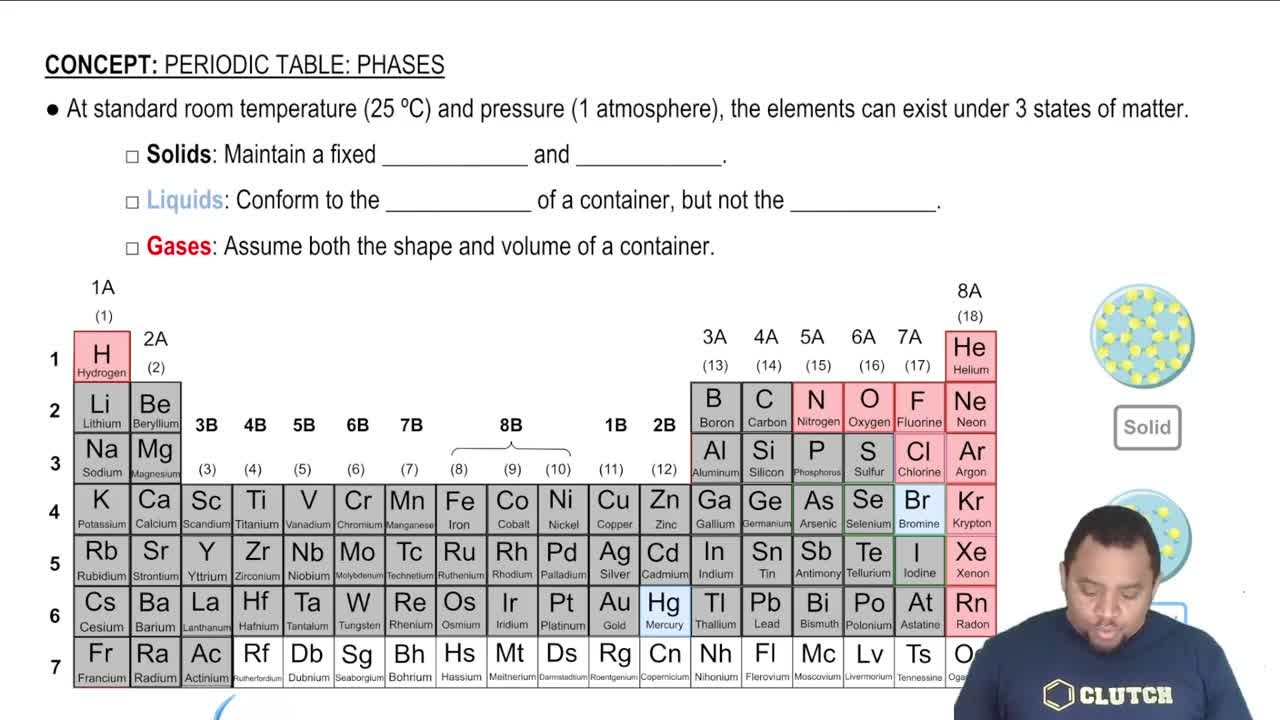
Element States of Matter
Related Practice
