A disproportionation reaction is an oxidation–reduction reaction in which the same substance is oxidized and reduced. Complete and balance the following disproportionation reactions:
(b) MnO42-(aq) → MnO4-(aq) + MnO2(s) (acidic solution)

 Verified step by step guidance
Verified step by step guidance
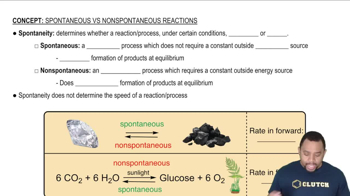

A disproportionation reaction is an oxidation–reduction reaction in which the same substance is oxidized and reduced. Complete and balance the following disproportionation reactions:
(b) MnO42-(aq) → MnO4-(aq) + MnO2(s) (acidic solution)
Gold exists in two common positive oxidation states, +1 and +3. The standard reduction potentials for these oxidation states are Au+1aq2 + e- ¡ Au1s2 Ered ° = +1.69 V Au3+1aq2 + 3 e- ¡ Au1s2 Ered ° = +1.50 V (c) Miners obtain gold by soaking gold-containing ores in an aqueous solution of sodium cyanide. A very soluble complex ion of gold forms in the aqueous solution because of the redox reaction 4 Au1s2 + 8 NaCN1aq2 + 2 H2O1l2 + O21g2 ¡ 4 Na3Au1CN2241aq2 + 4 NaOH1aq2 What is being oxidized, and what is being reduced in this reaction?