
A common shorthand way to represent a voltaic cell is anode | anode solution || cathode solution | cathode. A double vertical line represents a salt bridge or a porous barrier. A single vertical line represents a change in phase, such as from solid to solution. (a) Write the half-reactions and overall cell reaction represented by Fe | Fe2+ || Ag+ | Ag; calculate the standard cell emf using data in Appendix E. (b) Write the half-reactions and overall cell reaction represented by Zn | Zn2+ || H+ | H2; calculate the standard cell emf using data in Appendix E and use Pt for the hydrogen electrode. (c) Using the notation just described, represent a cell based on the following reaction: ClO3^-_(aq) + 3 Cu_(s) + 6 H+_(aq) -> Cl^-_(aq) + 3 Cu2+_(aq) + 3 H2O_(l); Pt is used as an inert electrode in contact with the ClO3^- and Cl^-. Calculate the standard cell emf given: ClO3^-_(aq) + 6 H+_(aq) + 6 e^- -> Cl^-_(aq) + 3 H2O_(l); E° = 1.45 V.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Voltaic Cells

Half-Reactions
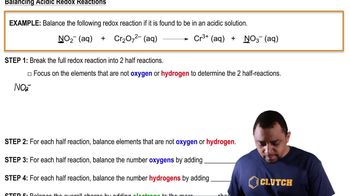
Standard Cell Potential (E°)
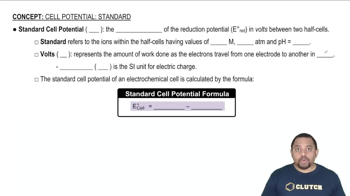
A disproportionation reaction is an oxidation–reduction reaction in which the same substance is oxidized and reduced. Complete and balance the following disproportionation reactions:
(b) MnO42-(aq) → MnO4-(aq) + MnO2(s) (acidic solution)
Predict whether the following reactions will be spontaneous in acidic solution under standard conditions: (a) oxidation of Sn to Sn2+ by I2 (to form I-), (b) reduction of Ni2+ to Ni by I- (to form I2), (c) reduction of Ce4+ to Ce3+ by H2O2, (d) reduction of Cu2+ to Cu by Sn2+ (to form Sn4+).
Gold exists in two common positive oxidation states, +1 and +3. The standard reduction potentials for these oxidation states are Au+1aq2 + e- ¡ Au1s2 Ered ° = +1.69 V Au3+1aq2 + 3 e- ¡ Au1s2 Ered ° = +1.50 V (c) Miners obtain gold by soaking gold-containing ores in an aqueous solution of sodium cyanide. A very soluble complex ion of gold forms in the aqueous solution because of the redox reaction 4 Au1s2 + 8 NaCN1aq2 + 2 H2O1l2 + O21g2 ¡ 4 Na3Au1CN2241aq2 + 4 NaOH1aq2 What is being oxidized, and what is being reduced in this reaction?
