In some applications nickel–cadmium batteries have been replaced by nickel–zinc batteries. The overall cell reaction for this relatively new battery is: 2 H2O(l) + 2 NiO(OH)(s) + Zn(s) → 2 Ni(OH)2(s) + Zn(OH)2(s) (c) A single nickel–cadmium cell has a voltage of 1.30 V. Based on the difference in the standard reduction potentials of Cd2+ and Zn2+, what voltage would you estimate a nickel–zinc battery will produce? (d) Would you expect the specific energy density of a nickel–zinc battery to be higher or lower than that of a nickel–cadmium battery?
Ch.20 - Electrochemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 20, Problem 78b
In some applications nickel–cadmium batteries have been replaced by nickel–zinc batteries. The overall cell reaction for this relatively new battery is: 2 H2O(l) + 2 NiO(OH)(s) + Zn(s) → 2 Ni(OH)2(s) + Zn(OH)2(s) (b) What is the anode half-reaction?
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants and products in the overall cell reaction to determine the changes occurring at each electrode.
Recognize that the anode is where oxidation occurs in a battery. Oxidation involves the loss of electrons.
Examine the overall reaction to find which reactant loses electrons. In this case, zinc (Zn) starts as a solid and ends up in a compound, indicating it likely undergoes oxidation.
Write the oxidation half-reaction for zinc. Start with the elemental form of zinc and show its transformation into its oxidized form, incorporating the appropriate number of electrons to balance the reaction.
Balance the half-reaction for mass and charge, ensuring the number of atoms and the electrical charges are equal on both sides of the equation.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
8mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Half-Reactions
Half-reactions are the individual reactions that occur at the anode and cathode in an electrochemical cell. They represent the oxidation and reduction processes separately. In the context of batteries, the anode half-reaction involves the loss of electrons, while the cathode half-reaction involves the gain of electrons. Understanding half-reactions is crucial for analyzing the overall cell reaction.
Recommended video:
Guided course
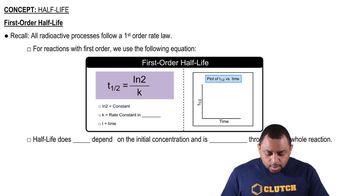
First-Order Half-Life
Oxidation and Reduction
Oxidation is the process where a substance loses electrons, while reduction is the gain of electrons. In electrochemical cells, the anode is where oxidation occurs, and the cathode is where reduction takes place. Identifying which species is oxidized and which is reduced helps in determining the half-reactions and understanding the flow of electrons in the circuit.
Recommended video:
Guided course
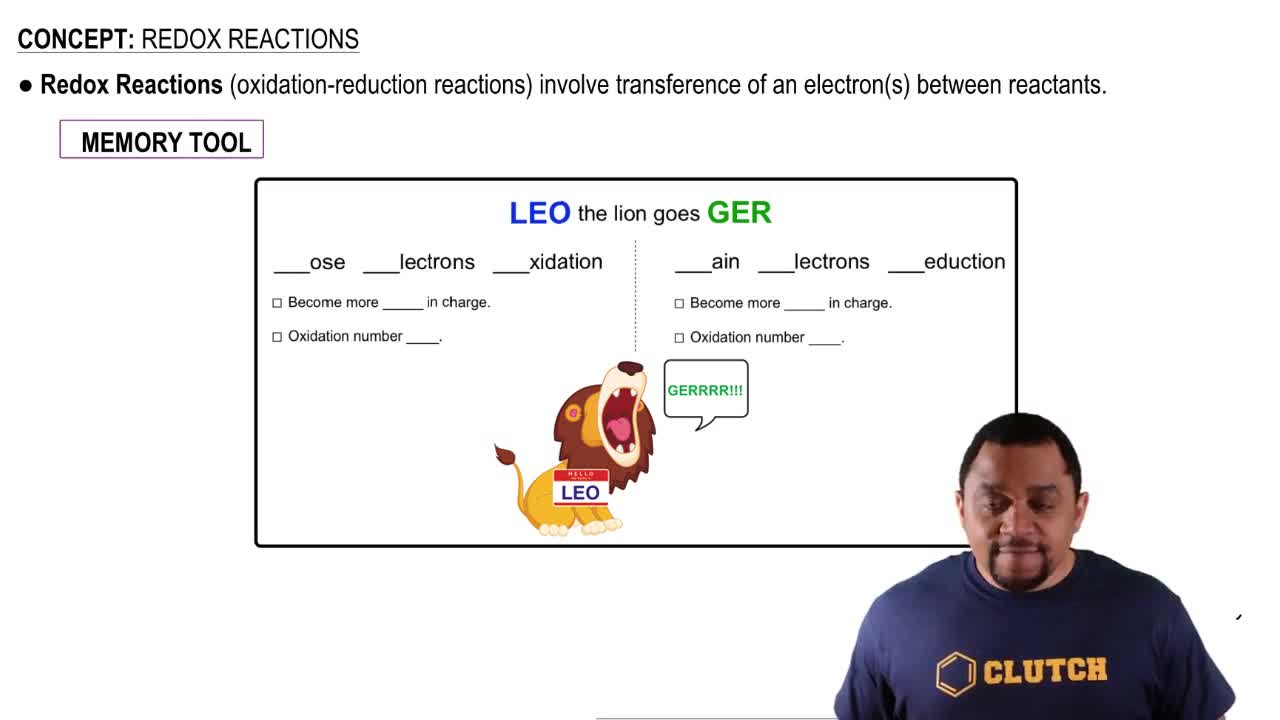
Oxidation and Reduction Reactions
Electrochemical Cells
Electrochemical cells convert chemical energy into electrical energy through redox reactions. They consist of two electrodes (anode and cathode) and an electrolyte. The flow of electrons from the anode to the cathode generates electric current. Knowing the structure and function of electrochemical cells is essential for analyzing battery reactions and their applications.
Recommended video:
Guided course
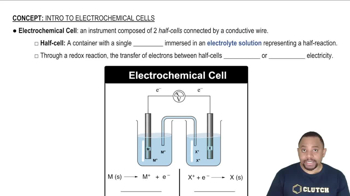
Electrochemical Cells
Related Practice
Textbook Question
Textbook Question
Heart pacemakers are often powered by lithium–silver chromate 'button' batteries. The overall cell reaction is 2 Li(s) + Ag2CrO4(s) → Li2CrO4(s) + 2 Ag(s) (b) Choose the two half-reactions from Appendix E that most closely approximate the reactions that occur in the battery. What standard emf would be generated by a voltaic cell based on these half-reactions?
Textbook Question
Li-ion batteries used in automobiles typically use a LiMn2O4 cathode in place of the LiCoO2 cathode found in most Li-ion batteries. (a) Calculate the mass percent lithium in each electrode material.
