Textbook Question
During the discharge of an alkaline battery, 4.50 g of Zn is consumed at the anode of the battery. (a) What mass of MnO2 is reduced at the cathode during this discharge?

 Verified step by step guidance
Verified step by step guidance
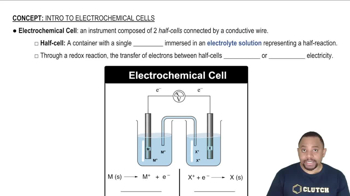
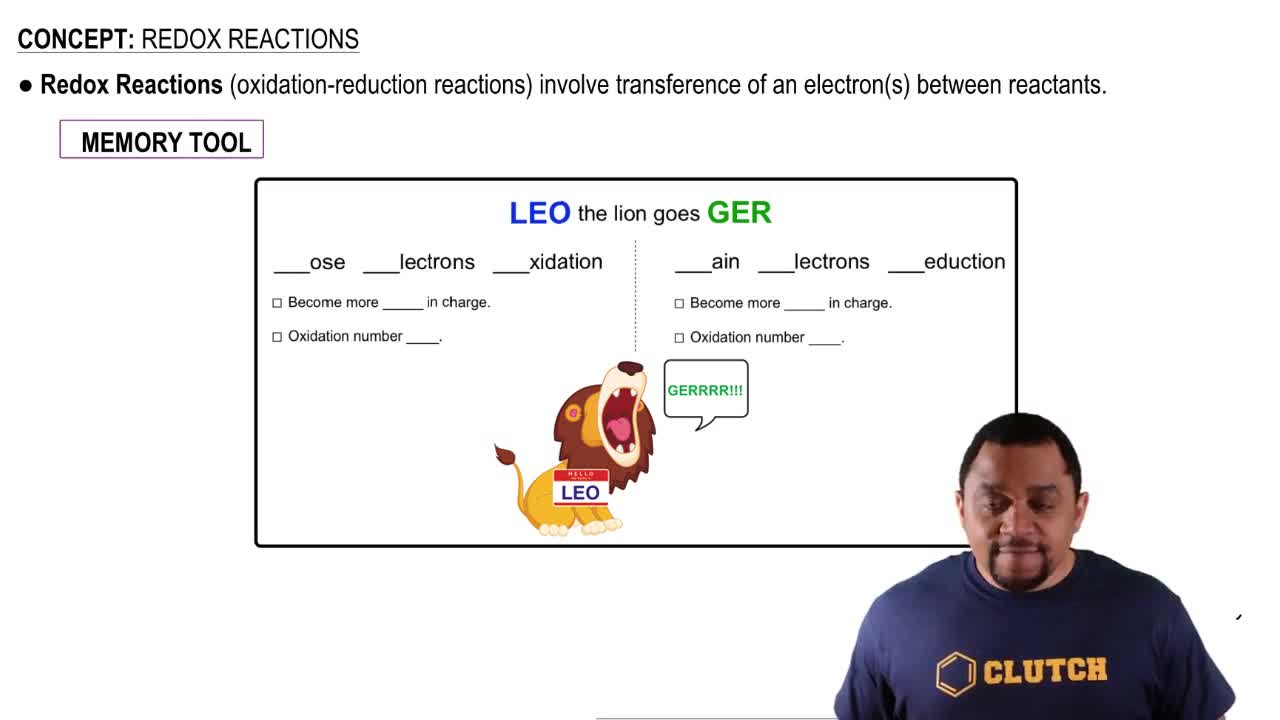

During the discharge of an alkaline battery, 4.50 g of Zn is consumed at the anode of the battery. (a) What mass of MnO2 is reduced at the cathode during this discharge?
During the discharge of an alkaline battery, 4.50 g of Zn is consumed at the anode of the battery. (b) How many coulombs of electrical charge are transferred from Zn to MnO2?
Heart pacemakers are often powered by lithium–silver chromate 'button' batteries. The overall cell reaction is 2 Li(s) + Ag2CrO4(s) → Li2CrO4(s) + 2 Ag(s) (b) Choose the two half-reactions from Appendix E that most closely approximate the reactions that occur in the battery. What standard emf would be generated by a voltaic cell based on these half-reactions?