Copper corrodes to cuprous oxide, Cu2O, or cupric oxide, CuO, depending on environmental conditions. (a) What is the oxidation state of copper in cuprous oxide?
Ch.20 - Electrochemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 20, Problem 89c
(c) What process occurs at the anode in the electrolysis of molten NaCl?
 Verified step by step guidance
Verified step by step guidance1
Identify the components involved in the electrolysis of molten NaCl: sodium ions (Na^+) and chloride ions (Cl^-).
Understand that electrolysis involves the movement of ions towards electrodes: cations move to the cathode and anions move to the anode.
Recognize that at the anode, oxidation occurs, which involves the loss of electrons by anions.
Write the half-reaction for the oxidation of chloride ions at the anode: \( 2\text{Cl}^- \rightarrow \text{Cl}_2 + 2e^- \).
Conclude that chlorine gas (Cl_2) is produced at the anode as a result of the oxidation of chloride ions.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolysis
Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous reaction. In this process, an electric current is passed through an electrolyte, causing the decomposition of the compound into its constituent elements. This is commonly used in the extraction of metals and the production of chemical compounds.
Recommended video:
Guided course

The Electrolytic Cell
Anode Reaction
The anode is the electrode where oxidation occurs during electrolysis. In the case of molten NaCl, chloride ions (Cl-) are oxidized at the anode, releasing chlorine gas (Cl2). This reaction is crucial as it illustrates the transfer of electrons and the conversion of ions into neutral atoms or molecules.
Recommended video:
Guided course
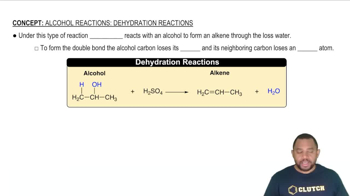
Alcohol Reactions: Dehydration Reactions
Ionic Compounds in Electrolysis
Ionic compounds, like sodium chloride (NaCl), dissociate into their respective ions when melted or dissolved in water. During electrolysis, these ions migrate towards the electrodes, where they undergo oxidation or reduction. Understanding the behavior of these ions is essential for predicting the products formed during the electrolysis process.
Recommended video:
Guided course

Ionic Compounds Naming
Related Practice
Textbook Question
Textbook Question
Copper corrodes to cuprous oxide, Cu2O, or cupric oxide, CuO, depending on environmental conditions. (c) Copper peroxide is another oxidation product of elemental copper. Suggest a formula for copper peroxide based on its name. (d) Copper(III) oxide is another unusual oxidation product of elemental copper. Suggest a chemical formula for copper(III) oxide.
Textbook Question
(d) Why is sodium metal not obtained when an aqueous solution of NaCl undergoes electrolysis?
Textbook Question
(d) Why are active metals such as Al obtained by electrolysis using molten salts rather than aqueous solutions?
Textbook Question
(a) A Cr3+(aq) solution is electrolyzed, using a current of 7.60 A. What mass of Cr(s) is plated out after 2.00 days?
