Textbook Question
Give the name or chemical formula, as appropriate, for each of the following acids: (b) HBr

 Verified step by step guidance
Verified step by step guidance
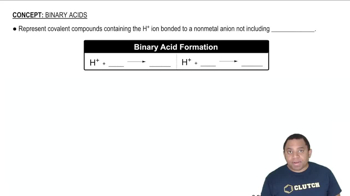

Give the name or chemical formula, as appropriate, for each of the following acids: (b) HBr
Give the name or chemical formula, as appropriate, for each of the following acids: (c) H3PO4
Give the name or chemical formula, as appropriate, for each of the following acids: (d) hypochlorous acid (e) iodic acid (f) sulfurous acid.
Provide the name or chemical formula, as appropriate, for each of the following acids: (e) HClO4
Provide the name or chemical formula, as appropriate, for each of the following acids: (f) CH3COOH.
Give the name or chemical formula, as appropriate, for each of the following binary molecular substances: (c) XeO3 (d) dinitrogen tetroxide (e) hydrogen cyanide