Massspectrometry is more often applied to molecules than to atoms. We will see in Chapter 3 that the molecular weight of a molecule is the sum of the atomic weights of the atoms in the molecule. The mass spectrum of H2 is taken under conditions that prevent decomposition into H atoms. The two naturally occurring isotopes of hydrogen are 1H (atomic mass = 1.00783 amu; abundance 99.9885%) and 2H (atomic mass = 2.01410; abundance 0.0115%). (a) How many peaks will the mass spectrum have?

Locate each of the following elements in the periodic table; give its name and atomic number, and indicate whether it is a metal, metalloid, or nonmetal: (a) Hg (b) At (c) Mo (d) W (e) Sn (f) V (g) K.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Periodic Table Organization
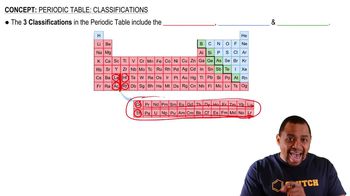
Classification of Elements
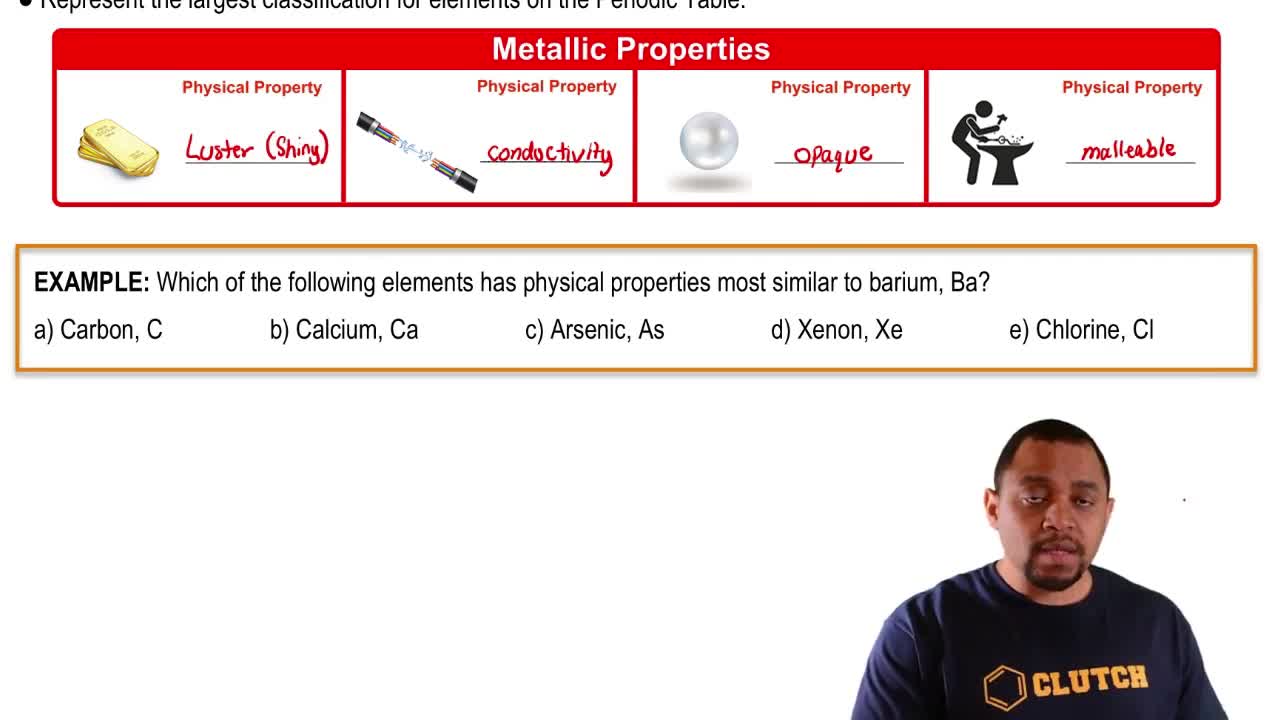
Atomic Number

Massspectrometry is more often applied to molecules than to atoms. We will see in Chapter 3 that the molecular weight of a molecule is the sum of the atomic weights of the atoms in the molecule. The mass spectrum of H2 is taken under conditions that prevent decomposition into H atoms. The two naturally occurring isotopes of hydrogen are 1H (atomic mass = 1.00783 amu; abundance 99.9885%) and 2H (atomic mass = 2.01410; abundance 0.0115%). (c) Which peak will be the largest, and which the smallest?
For each of the following elements, write its chemical symbol, locate it in the periodic table, give its atomic number, and indicate whether it is a metal, metalloid, or nonmetal: (a) radon (b) tellurium (c) cadmium (d) chromium (e) barium (f) selenium (g) arsenic.
For each of the following elements, write its chemical symbol, determine the name of the group to which it belongs (Table 2.3), and indicate whether it is a metal, metalloid, or nonmetal: (a) polonium (b) strontium (c) neon (d) rubidium (e) sulfur.
The structural formulas of the compounds n-butane and isobutane are shown below. (b) Determine the empirical formula of each.
