Massspectrometry is more often applied to molecules than to atoms. We will see in Chapter 3 that the molecular weight of a molecule is the sum of the atomic weights of the atoms in the molecule. The mass spectrum of H2 is taken under conditions that prevent decomposition into H atoms. The two naturally occurring isotopes of hydrogen are 1H (atomic mass = 1.00783 amu; abundance 99.9885%) and 2H (atomic mass = 2.01410; abundance 0.0115%). (a) How many peaks will the mass spectrum have?
Ch.2 - Atoms, Molecules, and Ions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 2, Problem 41
For each of the following elements, write its chemical symbol, locate it in the periodic table, give its atomic number, and indicate whether it is a metal, metalloid, or nonmetal: (a) radon (b) tellurium (c) cadmium (d) chromium (e) barium (f) selenium (g) arsenic.
 Verified step by step guidance
Verified step by step guidance1
Identify the chemical symbol for arsenic. The symbol for arsenic is As.
Locate arsenic in the periodic table. Arsenic is found in group 15 and period 4.
Determine the atomic number of arsenic. The atomic number of arsenic, which is the number of protons in its nucleus, is 33.
Classify arsenic as a metal, metalloid, or nonmetal. Arsenic is classified as a metalloid because it has properties intermediate between metals and nonmetals.
Summarize the information: Arsenic (As), located in group 15 and period 4 of the periodic table, has an atomic number of 33 and is a metalloid.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Periodic Table
The periodic table is a systematic arrangement of chemical elements, organized by increasing atomic number. Elements are grouped into rows (periods) and columns (groups) based on similar properties. Understanding the layout of the periodic table is essential for identifying elements, their symbols, and their classifications as metals, metalloids, or nonmetals.
Recommended video:
Guided course
Periodic Table Classifications
Atomic Number
The atomic number of an element is the number of protons found in the nucleus of its atoms, which determines the element's identity and its position in the periodic table. It also indicates the number of electrons in a neutral atom, influencing the element's chemical behavior and reactivity. For arsenic, the atomic number is 33.
Recommended video:
Guided course

Atom Structure
Classification of Elements
Elements are classified into three main categories: metals, metalloids, and nonmetals, based on their physical and chemical properties. Metals are typically shiny, conductive, and malleable; nonmetals are usually dull, insulative, and brittle; while metalloids exhibit properties of both. Arsenic, for example, is classified as a metalloid, which means it has characteristics of both metals and nonmetals.
Recommended video:
Guided course
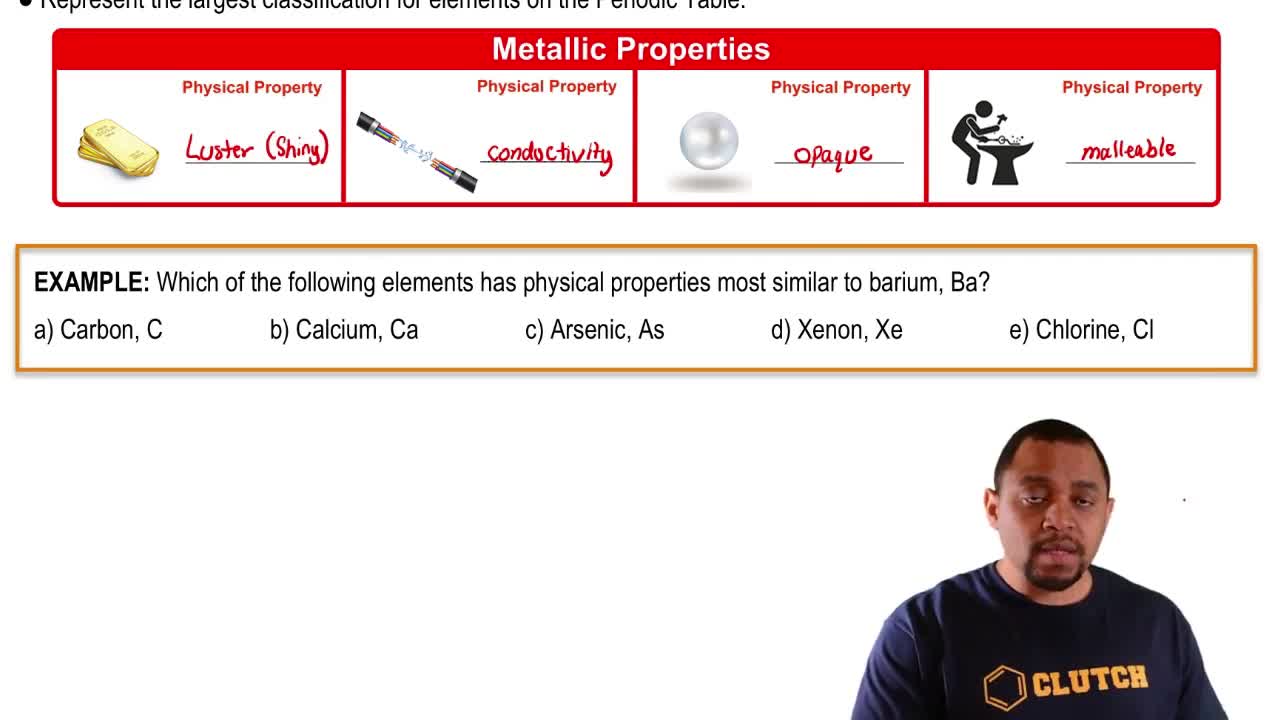
Element Classification Example
Related Practice
Textbook Question
Textbook Question
Massspectrometry is more often applied to molecules than to atoms. We will see in Chapter 3 that the molecular weight of a molecule is the sum of the atomic weights of the atoms in the molecule. The mass spectrum of H2 is taken under conditions that prevent decomposition into H atoms. The two naturally occurring isotopes of hydrogen are 1H (atomic mass = 1.00783 amu; abundance 99.9885%) and 2H (atomic mass = 2.01410; abundance 0.0115%). (c) Which peak will be the largest, and which the smallest?
Textbook Question
For each of the following elements, write its chemical symbol, determine the name of the group to which it belongs (Table 2.3), and indicate whether it is a metal, metalloid, or nonmetal: (a) polonium (b) strontium (c) neon (d) rubidium (e) sulfur.
1
views
