Textbook Question
Write the chemical formulas for the following compounds: (a) aluminum hydroxide (b) potassium sulfate (c) copper(I) oxide (d) zinc nitrate (e) mercury(II) bromide

 Verified step by step guidance
Verified step by step guidance


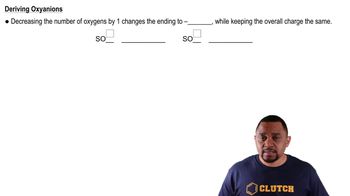
Write the chemical formulas for the following compounds: (a) aluminum hydroxide (b) potassium sulfate (c) copper(I) oxide (d) zinc nitrate (e) mercury(II) bromide
Write the chemical formulas for the following compounds: (f) iron(III)carbonate
Write the chemical formulas for the following compounds: (g) sodium hypobromite.
Give the chemical formula for each of the following ionic compounds: (e) cobalt(II) hydrogen carbonate (f) chromium(III) acetate (g) potassium dichromate.
Give the name or chemical formula, as appropriate, for each of the following acids: (b) HBr
Give the name or chemical formula, as appropriate, for each of the following acids: (c) H3PO4