The radius of an atom of copper (Cu) is about 140 pm. (a) Express this distance in angstroms 1A 2.

Answer the following questions without referring to Table 2.1: (a) What are the main subatomic particles that make up the atom? (b) What is the relative charge (in multiples of the electronic charge) of each proton? (b) What is the relative charge (in multiples of the electronic charge) of each neutron? (b) What is the relative charge (in multiples of the electronic charge) of each electron? (c) Which of the particles is the most massive? (d) Which is the least massive?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Subatomic Particles
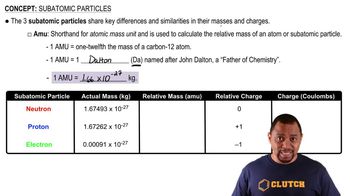
Relative Charge of Subatomic Particles

Mass of Subatomic Particles

The radius of an atom of copper (Cu) is about 140 pm. (b) How many Cu atoms would have to be placed side by side to span a distance of 5.0 mm?
The radius of an atom of copper (Cu) is about 140 pm. (c) If you assume that the Cu atom is a sphere, what is the volume in cm3 of a single atom?
Determine whether each of the following statements is true or false. If false, correct the statement to make it true: (a) The nucleus has most of the mass and comprises most of the volume of an atom.
Determine whether each of the following statements is true or false. If false, correct the statement to make it true: (b) Every atom of a given element has the same number of protons.
Determine whether each of the following statements is true or false. If false, correct the statement to make it true: (c) The number of electrons in an atom equals the number of neutrons in the atom.
