Determine whether each of the following statements is true or false. If false, correct the statement to make it true: (a) The nucleus has most of the mass and comprises most of the volume of an atom.
Ch.2 - Atoms, Molecules, and Ions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 2, Problem 22c
Determine whether each of the following statements is true or false. If false, correct the statement to make it true: (c) The number of electrons in an atom equals the number of neutrons in the atom.
 Verified step by step guidance
Verified step by step guidance1
Understand the structure of an atom: An atom consists of protons, neutrons, and electrons.
Recall that the number of protons in an atom is equal to the atomic number, and in a neutral atom, the number of electrons equals the number of protons.
Recognize that the number of neutrons can vary even among atoms of the same element, leading to different isotopes.
Identify that the statement 'The number of electrons in an atom equals the number of neutrons in the atom' is generally false.
Correct the statement: In a neutral atom, the number of electrons equals the number of protons, not necessarily the number of neutrons.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Atomic Structure
Atoms are composed of three primary subatomic particles: protons, neutrons, and electrons. Protons and neutrons reside in the nucleus, while electrons orbit around the nucleus. The number of protons defines the element, while the number of electrons typically equals the number of protons in a neutral atom, ensuring electrical neutrality.
Recommended video:
Guided course

Atom Structure
Electrons vs. Neutrons
Electrons and neutrons serve different roles in an atom. Electrons are negatively charged and are involved in chemical bonding and reactions, while neutrons are neutral particles that contribute to the atomic mass and stability of the nucleus. The number of neutrons can vary in isotopes of an element, unlike electrons, which are usually equal to protons in neutral atoms.
Recommended video:
Guided course
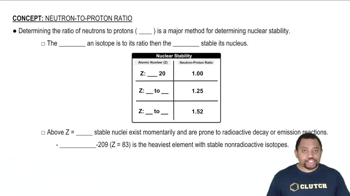
Neutron-Proton Ratio
True/False Statements in Science
In scientific reasoning, statements can often be evaluated as true or false based on established facts. When a statement is false, it is important to identify the error and provide a corrected version. In this case, the statement incorrectly equates the number of electrons with neutrons, which can be corrected by stating that the number of electrons equals the number of protons in a neutral atom.
Recommended video:
Guided course

Third Law of Thermodynamics Example
Related Practice
Textbook Question
7
views
1
rank
Textbook Question
Determine whether each of the following statements is true or false. If false, correct the statement to make it true: (b) Every atom of a given element has the same number of protons.
14
views
Textbook Question
Determine whether each of the following statements is true or false. If false, correct the statement to make it true: (d) The protons in the nucleus of the helium atom are held together by a force called the strong nuclear force.
27
views
1
rank
Textbook Question
Consider an atom of 10B. (a) How many protons, neutrons, and electrons does this atom contain?
3
views
Textbook Question
Consider an atom of 10B. (b) What is the symbol of the atom obtained by adding one proton to 10B?
4
views
