Textbook Question
Use data in Appendix C to calculate ΔH°, ΔS°, and ΔG° at 25 °C for each of the following reactions.
a. 4 Cr(s) + 3 O2(g) → 2 Cr2O3(s)
b. BaCO3(s) → BaO(s) + CO2(g)
c. 2 P(s) + 10 HF(g) → 2 PF5(g) + 5 H2(g)
d. K(s) + O2(g) → KO2(s)

 Verified step by step guidance
Verified step by step guidance
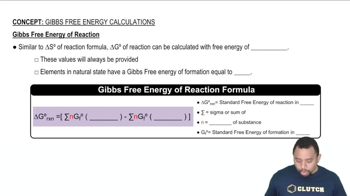

Use data in Appendix C to calculate ΔH°, ΔS°, and ΔG° at 25 °C for each of the following reactions.
a. 4 Cr(s) + 3 O2(g) → 2 Cr2O3(s)
b. BaCO3(s) → BaO(s) + CO2(g)
c. 2 P(s) + 10 HF(g) → 2 PF5(g) + 5 H2(g)
d. K(s) + O2(g) → KO2(s)
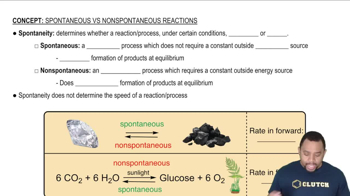
Using data from Appendix C, calculate ΔG° for the following reactions. Indicate whether each reaction is spontaneous at 298 K under standard conditions.
(a) 2 SO2(g) + O2(g) → 2 SO3(g)
(b) NO2(g) + N2O(g) → 3 NO(g)
(c) 6 Cl2(g) + 2 Fe2O3(s) → 4 FeCl3(s) + 3 O2(g)
(d) SO2(g) + 2 H2(g) → S(s) + 2 H2O(g)
Sulfur dioxide reacts with strontium oxide as follows: SO2(g) + SrO(g) → SrSO3(s) (a) Without using thermochemical data, predict whether ΔG° for this reaction is more negative or less negative than ΔH°.