For a certain chemical reaction, ΔH° = -35.4 kJ and ΔS° = -85.5 J/K. (c) Calculate ΔG° for the reaction at 298 K. (d) Is the reaction spontaneous at 298 K under standard conditions?
Ch.19 - Chemical Thermodynamics

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 19, Problem 58
Use data in Appendix C to calculate ΔH°, ΔS°, and ΔG° at 25 °C for each of the following reactions.
a. 4 Cr(s) + 3 O2(g) → 2 Cr2O3(s)
b. BaCO3(s) → BaO(s) + CO2(g)
c. 2 P(s) + 10 HF(g) → 2 PF5(g) + 5 H2(g)
d. K(s) + O2(g) → KO2(s)
 Verified step by step guidance
Verified step by step guidance1
1. To calculate the ΔH° (standard enthalpy change), ΔS° (standard entropy change), and ΔG° (standard Gibbs free energy change) for the reaction, you need to use the standard enthalpy, entropy, and Gibbs free energy of formation values from Appendix C for each substance involved in the reaction. The standard enthalpy, entropy, and Gibbs free energy of a reaction are calculated using the formula: ΔH° = Σ ΔHf°(products) - Σ ΔHf°(reactants), ΔS° = Σ S°(products) - Σ S°(reactants), and ΔG° = ΔH° - TΔS°, respectively, where T is the absolute temperature in Kelvin (25 °C = 298.15 K).
2. For ΔH°, look up the standard enthalpy of formation (ΔHf°) for each substance in the reaction in Appendix C. Multiply each ΔHf° by the stoichiometric coefficient in the balanced chemical equation and add up the values for the products and reactants separately. Then subtract the sum for the reactants from the sum for the products.
3. For ΔS°, look up the standard molar entropy (S°) for each substance in the reaction in Appendix C. Multiply each S° by the stoichiometric coefficient in the balanced chemical equation and add up the values for the products and reactants separately. Then subtract the sum for the reactants from the sum for the products.
4. For ΔG°, use the ΔH° and ΔS° values you calculated and the temperature in Kelvin. Substitute these values into the equation ΔG° = ΔH° - TΔS° to calculate ΔG°.
5. Remember to check the units. The standard enthalpy and Gibbs free energy of formation are usually given in kJ/mol, while the standard molar entropy is given in J/(mol·K). You may need to convert units to make them consistent.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Enthalpy (ΔH°)
Enthalpy, represented as ΔH°, is a measure of the total heat content of a system at constant pressure. It reflects the energy absorbed or released during a chemical reaction. A negative ΔH° indicates an exothermic reaction, while a positive ΔH° signifies an endothermic reaction. Understanding how to calculate ΔH° using standard enthalpies of formation is crucial for evaluating the energy changes in the given reaction.
Recommended video:
Guided course

Enthalpy of Formation
Entropy (ΔS°)
Entropy, denoted as ΔS°, is a measure of the disorder or randomness in a system. It quantifies the number of ways a system can be arranged, with higher entropy indicating greater disorder. In chemical reactions, changes in entropy can influence the spontaneity of the reaction. Calculating ΔS° involves using standard molar entropies, which helps in understanding how the reaction's disorder changes from reactants to products.
Recommended video:
Guided course
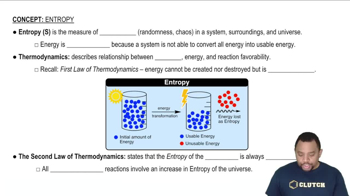
Entropy in Thermodynamics
Gibbs Free Energy (ΔG°)
Gibbs Free Energy, represented as ΔG°, combines enthalpy and entropy to determine the spontaneity of a reaction at constant temperature and pressure. The relationship ΔG° = ΔH° - TΔS° indicates that a negative ΔG° suggests a spontaneous reaction, while a positive ΔG° indicates non-spontaneity. Calculating ΔG° is essential for predicting whether the reaction will occur under standard conditions, such as at 25 °C.
Recommended video:
Guided course
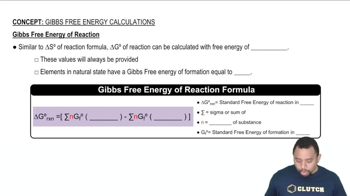
Gibbs Free Energy of Reactions
Related Practice
Textbook Question
Textbook Question
Using data from Appendix C, calculate ΔG° for the following reactions. Indicate whether each reaction is spontaneous at 298 K under standard conditions.
(a) 2 SO2(g) + O2(g) → 2 SO3(g)
(b) NO2(g) + N2O(g) → 3 NO(g)
(c) 6 Cl2(g) + 2 Fe2O3(s) → 4 FeCl3(s) + 3 O2(g)
(d) SO2(g) + 2 H2(g) → S(s) + 2 H2O(g)
Textbook Question
Using data from Appendix C, calculate the change in Gibbs free energy for each of the following reactions. In each case, indicate whether the reaction is spontaneous at 298 K under standard conditions.
(a) 2 Ag(s) + Cl2(g) → 2 AgCl(s)
(b) P4O10(s) + 16 H2(g) → 4 PH3(g) + 10 H2O(g)
(c) CH4(g) + 4 F2(g) → CF4(g) + 4 HF(g)
(d) 2 H2O2(l) → 2 H2O(l) + O2(g)
