You have to prepare a pH = 3.50 buffer, and you have the following 0.10 M solutions available: HCOOH, CH3COOH, H3PO4, HCOONa, CH3COONa, and NaH2PO4. Which solutions would you use?

You have to prepare a pH = 5.00 buffer, and you have the following 0.10 M solutions available: HCOOH, HCOONa, CH3COOH, CH3COONa, HCN, and NaCN. How many milliliters of each solution would you use to make approximately 1 L of the buffer?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Buffer Solutions

Henderson-Hasselbalch Equation

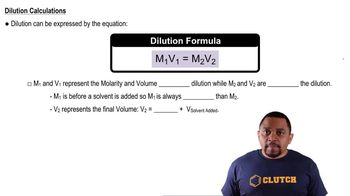
Concentration and Dilution
You have to prepare a pH = 3.50 buffer, and you have the following 0.10 M solutions available: HCOOH, CH3COOH, H3PO4, HCOONa, CH3COONa, and NaH2PO4. How many milliliters of each solution would you use to make approximately 1 L of the buffer?
You have to prepare a pH = 5.00 buffer, and you have the following 0.10 M solutions available: HCOOH, HCOONa, CH3COOH, CH3COONa, HCN, and NaCN. Which solutions would you use?
The accompanying graph shows the titration curves for two monoprotic acids. (d) Estimate the pKa of the weak acid.
Compare the titration of a strong, monoprotic acid with a strong base to the titration of a weak, monoprotic acid with a strong base. Assume the strong and weak acid solutions initially have the same concentrations. Indicate whether the following statements are true or false. (a) More base is required to reach the equivalence point for the strong acid than the weak acid.
