(a) What is the ratio of HCO3- to H2CO3 in blood of pH 7.4?

You have to prepare a pH = 3.50 buffer, and you have the following 0.10 M solutions available: HCOOH, CH3COOH, H3PO4, HCOONa, CH3COONa, and NaH2PO4. How many milliliters of each solution would you use to make approximately 1 L of the buffer?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Buffer Solutions

Henderson-Hasselbalch Equation

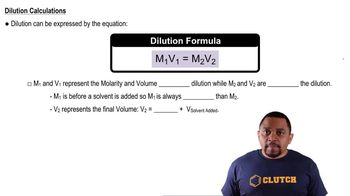
Concentration and Dilution
(b) What is the ratio of HCO3- to H2CO3 in an exhausted marathon runner whose blood pH is 7.1?
You have to prepare a pH = 3.50 buffer, and you have the following 0.10 M solutions available: HCOOH, CH3COOH, H3PO4, HCOONa, CH3COONa, and NaH2PO4. Which solutions would you use?
You have to prepare a pH = 5.00 buffer, and you have the following 0.10 M solutions available: HCOOH, HCOONa, CH3COOH, CH3COONa, HCN, and NaCN. Which solutions would you use?
You have to prepare a pH = 5.00 buffer, and you have the following 0.10 M solutions available: HCOOH, HCOONa, CH3COOH, CH3COONa, HCN, and NaCN. How many milliliters of each solution would you use to make approximately 1 L of the buffer?
The accompanying graph shows the titration curves for two monoprotic acids. (d) Estimate the pKa of the weak acid.
