Lead(II) carbonate, PbCO3, is one of the components of the passivating layer that forms inside lead pipes. (d) The EPA threshold for acceptable levels of lead ions in water is 15 ppb. Does a saturated solution of lead(II) carbonate produce a solution that exceeds the EPA limit?
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 17, Problem 99b
The solubility of CaCO3 is pH dependent. (b) Use the Kb expression for the CO32- ion to determine the equilibrium constant for the reaction CaCO3(s) + H2O(l) ⇌ Ca2+(aq) + HCO3-(aq) + OH-(aq)
 Verified step by step guidance
Verified step by step guidance1
Write the balanced chemical equation for the dissolution of CaCO3 in water: CaCO3(s) + H2O(l) ⇌ Ca^2+(aq) + HCO3^-(aq) + OH^-(aq).
Identify the Kb expression for the CO3^2- ion, which is the base dissociation constant for the reaction CO3^2-(aq) + H2O(l) ⇌ HCO3^-(aq) + OH^-(aq).
Use the Kb expression to express the concentrations of the products and reactants at equilibrium. The expression is Kb = [HCO3^-][OH^-] / [CO3^2-].
Combine the dissolution equation of CaCO3 and the Kb expression to find the overall equilibrium expression. Since CaCO3 is a solid, its concentration does not appear in the expression.
Solve for the equilibrium constant (K) of the overall reaction by incorporating the stoichiometry and the constants from the Kb expression. This will involve algebraic manipulation to express K in terms of known quantities and constants.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility Product Constant (Ksp)
The solubility product constant (Ksp) is an equilibrium constant that applies to the solubility of sparingly soluble ionic compounds. It represents the product of the molar concentrations of the ions, each raised to the power of their coefficients in the balanced equation. For calcium carbonate (CaCO3), Ksp can be used to understand how its solubility changes with pH, as the formation of bicarbonate and carbonate ions affects the equilibrium.
Recommended video:
Guided course

Solubility Product Constant
Base Ionization Constant (Kb)
The base ionization constant (Kb) is a measure of the strength of a base in solution, indicating how well it can accept protons (H+) from water. For the carbonate ion (CO32-), Kb can be used to derive the equilibrium constant for its reaction with water, producing bicarbonate (HCO3-) and hydroxide ions (OH-). This relationship is crucial for understanding the pH dependence of CaCO3 solubility.
Recommended video:
Guided course
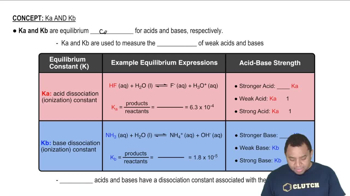
Characteristics of Ka and Kb
Le Chatelier's Principle
Le Chatelier's Principle states that if a system at equilibrium is subjected to a change in concentration, temperature, or pressure, the system will adjust to counteract that change and restore a new equilibrium. In the context of CaCO3 solubility, changes in pH (which affect the concentration of H+ ions) will shift the equilibrium position, influencing the solubility of CaCO3 and the formation of its ions in solution.
Recommended video:
Guided course

Le Chatelier's Principle
Related Practice
Textbook Question
Textbook Question
For each pair of compounds, use Ksp values to determine which has the greater molar solubility: (a) CdS or CuS (b) PbCO3 or BaCrO4 (c) Ni(OH)2 or NiCO3 (d) AgI or Ag2SO4.
Textbook Question
Tooth enamel is composed of hydroxyapatite, whose simplest formula is Ca51PO423OH, and whose corresponding Ksp = 6.8 * 10-27. As discussed in the Chemistry and Life box on page 746, fluoride in fluorinated water or in toothpaste reacts with hydroxyapatite to form fluoroapatite, Ca51PO423F, whose Ksp = 1.0 * 10-60. (a) Write the expression for the solubility-constant for hydroxyapatite and for fluoroapatite.
Textbook Question
Calculate the solubility of Mg1OH22 in 0.50 M NH4Cl.
