Suppose you want to do a physiological experiment that calls for a pH 6.50 buffer. You find that the organism with which you are working is not sensitive to the weak acid H2A 1Ka1 = 2 * 10-2; Ka2 = 5.0 * 10-72 or its sodium salts. You have available a 1.0 M solution of this acid and a 1.0 M solution of NaOH. How much of the NaOH solution should be added to 1.0 L of the acid to give a buffer at pH 6.50? (Ignore any volume change.)
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 17, Problem 97
Lead(II) carbonate, PbCO3, is one of the components of the passivating layer that forms inside lead pipes. (a) If the Ksp for PbCO3 is 7.4 * 10^-14, what is the molarity of Pb2+ in a saturated solution of lead(II) carbonate? (b) What is the concentration in ppb of Pb2+ ions in a saturated solution?
 Verified step by step guidance
Verified step by step guidance1
To find the molarity of Pb²⁺ in a saturated solution of PbCO₃, start by writing the dissolution equation: PbCO₃(s) ⇌ Pb²⁺(aq) + CO₃²⁻(aq).
The solubility product constant (Ksp) expression for this equilibrium is Ksp = [Pb²⁺][CO₃²⁻]. Since the stoichiometry of the dissolution is 1:1, let the solubility of PbCO₃ be 's'. Then, [Pb²⁺] = s and [CO₃²⁻] = s.
Substitute the expressions for the ion concentrations into the Ksp expression: Ksp = s * s = s². Given that Ksp = 7.4 * 10⁻¹⁴, set up the equation s² = 7.4 * 10⁻¹⁴.
Solve for 's' to find the molarity of Pb²⁺ in the saturated solution. This involves taking the square root of both sides of the equation.
To find the concentration of Pb²⁺ in parts per billion (ppb), use the formula: concentration in ppb = (molarity of Pb²⁺) * (molar mass of Pb) * 10⁶. Calculate the molar mass of Pb and substitute the values to find the concentration in ppb.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility Product Constant (Ksp)
The solubility product constant (Ksp) is an equilibrium constant that applies to the solubility of sparingly soluble ionic compounds. It is defined as the product of the molar concentrations of the ions, each raised to the power of their coefficients in the balanced equation. For lead(II) carbonate, PbCO3, the Ksp expression is Ksp = [Pb2+][CO3^2-]. This value helps determine the maximum concentration of ions in a saturated solution.
Recommended video:
Guided course

Solubility Product Constant
Saturated Solution
A saturated solution is a solution that contains the maximum concentration of a solute that can dissolve at a given temperature and pressure. In the case of PbCO3, a saturated solution means that the concentrations of Pb2+ and CO3^2- ions remain constant, as the rate of dissolution equals the rate of precipitation. Understanding this concept is crucial for calculating the molarity of Pb2+ in the solution.
Recommended video:
Guided course
Saturated and Unsaturated Hydrocarbons
Parts Per Billion (ppb)
Parts per billion (ppb) is a unit of measurement used to describe the concentration of a substance in a solution, indicating how many parts of the substance are present in one billion parts of the solution. To convert molarity to ppb, one must consider the molar mass of the solute and the density of the solution. This concept is essential for expressing the concentration of Pb2+ ions in a way that is relevant for environmental and health assessments.
Recommended video:
Guided course
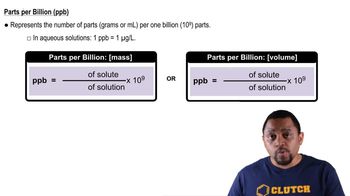
Parts per Billion (ppb)
Related Practice
Textbook Question
Textbook Question
Lead(II) carbonate, PbCO3, is one of the components of the passivating layer that forms inside lead pipes. (d) The EPA threshold for acceptable levels of lead ions in water is 15 ppb. Does a saturated solution of lead(II) carbonate produce a solution that exceeds the EPA limit?
Textbook Question
For each pair of compounds, use Ksp values to determine which has the greater molar solubility: (a) CdS or CuS (b) PbCO3 or BaCrO4 (c) Ni(OH)2 or NiCO3 (d) AgI or Ag2SO4.
