Two buffers are prepared by adding an equal number of moles of formic acid (HCOOH) and sodium formate (HCOONa) to enough water to make 1.00 L of solution. Buffer A is prepared using 1.00 mol each of formic acid and sodium formate. Buffer B is prepared by using 0.010 mol of each. (b) Which buffer will have the greater buffer capacity?

A biochemist needs 750 mL of an acetic acid–sodium acetate buffer with pH 4.50. Solid sodium acetate (CH3COONa) and glacial acetic acid (CH3COOH) are available. Glacial acetic acid is 99% CH3COOH by mass and has a density of 1.05 g/mL. If the buffer is to be 0.15 M in CH3COOH, how many grams of CH3COONa and how many milliliters of glacial acetic acid must be used?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Buffer Solutions

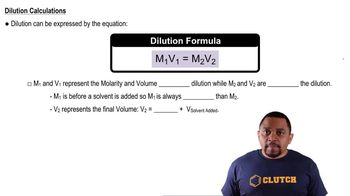
Molarity and Dilution
Stoichiometry

A sample of 0.2140 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.0950 M NaOH. The acid required 30.0 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?
A sample of 0.1687 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.1150 M NaOH. The acid required 15.5 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?
A sample of 0.1687 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.1150 M NaOH. The acid required 15.5 mL of base to reach the equivalence point. (b) After 7.25 mL of base had been added in the titration, the pH was found to be 2.85. What is the Ka for the unknown acid?
