Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (b) Using Appendix D, calculate the Ka for pyridinium bromide.
Ch.16 - Acid-Base Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 16, Problem 83c
Predict whether aqueous solutions of the following compounds are acidic, basic, or neutral: (c) Na2CO3
 Verified step by step guidance
Verified step by step guidance1
Identify the ions formed when Na2CO3 dissolves in water: Na2CO3 dissociates into 2 Na^+ ions and 1 CO3^2- ion.
Recognize that Na^+ is a spectator ion, which does not affect the acidity or basicity of the solution.
Focus on the CO3^2- ion, which is the conjugate base of the weak acid H2CO3 (carbonic acid).
Understand that CO3^2- can react with water in a hydrolysis reaction to form HCO3^- and OH^-: CO3^2- + H2O ⇌ HCO3^- + OH^-.
Conclude that the presence of OH^- ions makes the solution basic.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Acid-Base Theory
Acid-base theory explains the behavior of substances in terms of their ability to donate protons (H+) or accept protons. According to the Brønsted-Lowry theory, acids are proton donors, while bases are proton acceptors. Understanding this theory is essential for predicting the acidity or basicity of a solution.
Recommended video:
Guided course

Bronsted-Lowry Acid-Base Theory
Salt Hydrolysis
Salt hydrolysis occurs when an ionic compound dissolves in water and its ions interact with water molecules, potentially affecting the pH of the solution. In the case of Na2CO3, the carbonate ion (CO3^2-) can react with water to produce hydroxide ions (OH-), leading to a basic solution. This concept is crucial for determining the nature of the solution.
Recommended video:
Guided course
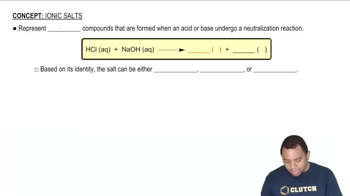
Ionic Salts
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 (very acidic) to 14 (very basic), with 7 being neutral. A pH less than 7 indicates an acidic solution, while a pH greater than 7 indicates a basic solution. Understanding the pH scale helps in predicting the behavior of solutions, such as those formed by Na2CO3.
Recommended video:
Guided course

The pH Scale
Related Practice
Textbook Question
Textbook Question
Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (c) A solution of pyridinium bromide has a pH of 2.95. What is the concentration of the pyridinium cation at equilibrium, in units of molarity?
Textbook Question
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (a) AlCl3
Textbook Question
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (b) NaBr
Textbook Question
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (c) NaClO
1
views
