Textbook Question
The fluoride ion reacts with water to produce HF. (c) Is fluoride acting as a Lewis acid or as a Lewis base when reacting with water?

 Verified step by step guidance
Verified step by step guidance
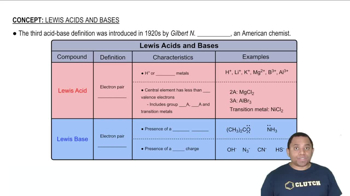
Identify the Lewis acid and Lewis base among the reactants in each of the following reactions:
(a) Fe(ClO4)3(s) + 6 H2O(l) ⇌ [Fe(H2O)6]3+(aq) + 3 ClO4-(aq)
(b) CN-(aq) + H2O(l) ⇌ HCN(aq) + OH-(aq)
(c) (CH3)3N(g) + BF3(g) ⇌ (CH3)NBF3(s)
(d) HIO(lq) + NH2-(lq) ⇌ NH3(lq) + IO-(lq) (lq denotes liquid ammonia as solvent)
Which member of each pair produces the more acidic aqueous solution: (a) ZnBr2 or CdCl2 (b) CuCl or Cu(NO3)2 (c) Ca(NO3)2 or NiBr2