The following equilibria were measured at 823 K: CoO(s) + H2(g) ⇌ Co(s) + H2O(g) Kc = 67 H2(g) + CO2(g) ⇌ CO(g) + H2O(g) Kc = 0.14 (d) If the reaction vessel from part (c) is heated to 823 K and allowed to come to equilibrium, how much CoO(s) remains?

At 800 K, the equilibrium constant for the reaction A2(g) ⇌ 2 A(g) is Kc = 3.1 × 10-4. (d) If the temperature is raised to 1000 K, will the reverse rate constant kr increase or decrease? Will the change in kr be larger or smaller than the change in kf?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Equilibrium Constant (Kc)

Rate Constants (kf and kr)
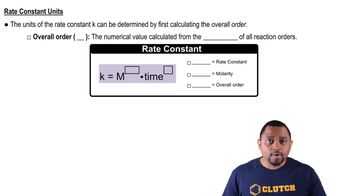
Temperature Dependence of Reaction Rates
The phase diagram for SO2 is shown here. (d) At which of the three points marked in red does SO2(g) most closely approach ideal-gas behavior?
The phase diagram for SO2 is shown here. (e) At which of the three red points does SO2(g) behave least ideally?
In Section 11.5, we defined the vapor pressure of a liquid in terms of an equilibrium. (a) Write the equation representing the equilibrium between liquid water and water vapor and the corresponding expression for Kp.
In Section 11.5, we defined the vapor pressure of a liquid in terms of an equilibrium. (b) By using data in Appendix B, give the value of Kp for this reaction at 30 C.
In Section 11.5, we defined the vapor pressure of a liquid in terms of an equilibrium. (c) What is the value of Kp for any liquid in equilibrium with its vapor at the normal boiling point of the liquid?
