Textbook Question
The mechanism for the oxidation of HBr by O2 to form 2 H2O and Br2 is shown in Exercise 14.74. (a) Calculate the overall standard enthalpy change for the reaction process.


 Verified step by step guidance
Verified step by step guidance


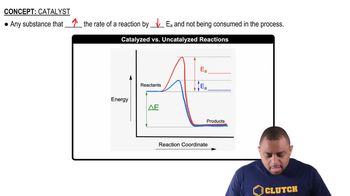
The mechanism for the oxidation of HBr by O2 to form 2 H2O and Br2 is shown in Exercise 14.74. (a) Calculate the overall standard enthalpy change for the reaction process.
The mechanism for the oxidation of HBr by O2 to form 2 H2O and Br2 is shown in Exercise 14.74. (c) Draw a plausible Lewis structure for the intermediate HOOBr. To what familiar compound of hydrogen and oxygen does it appear similar?
Many primary amines, RNH2, where R is a carboncontaining fragment such as CH3, CH3CH2, and so on, undergo reactions where the transition state is tetrahedral. (a) Draw a hybrid orbital picture to visualize the bonding at the nitrogen in a primary amine (just use a C atom for 'R').