The reaction between ethyl iodide and hydroxide ion in ethanol (C2H5OH) solution, C2H5I(alc) + OH-(alc) → C2H5OH(l) + I-(alc), has an activation energy of 86.8 kJ/mol and a frequency factor of 2.10 × 1011 M-1 s-1. (d) Assuming the frequency factor and activation energy do not change as a function of temperature, calculate the rate constant for the reaction at 50 C.

The mechanism for the oxidation of HBr by O2 to form 2 H2O and Br2 is shown in Exercise 14.74. (a) Calculate the overall standard enthalpy change for the reaction process.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Standard Enthalpy Change

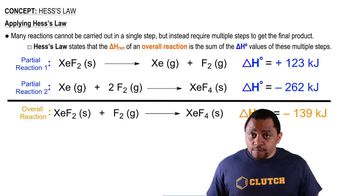
Hess's Law
Bond Enthalpies

The gas-phase reaction of NO with F2 to form NOF and F has an activation energy of Ea = 6.3 kJ/mol. and a frequency factor of A = 6.0 × 108 M-1 s-1. The reaction is believed to be bimolecular: NO(g) + F2(g) → NOF(g) + F(g) (b) Draw the Lewis structures for the NO and the NOF molecules, given that the chemical formula for NOF is misleading because the nitrogen atom is actually the central atom in the molecule.
The gas-phase reaction of NO with F2 to form NOF and F has an activation energy of Ea = 6.3 kJ>mol. and a frequency factor of A = 6.0 * 108 M-1 s-1. The reaction is believed to be bimolecular: NO1g2 + F21g2 ¡ NOF1g2 + F1g2 (e) Suggest a reason for the low activation energy for the reaction.
The mechanism for the oxidation of HBr by O2 to form 2 H2O and Br2 is shown in Exercise 14.74. (c) Draw a plausible Lewis structure for the intermediate HOOBr. To what familiar compound of hydrogen and oxygen does it appear similar?
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride and hydrogen chloride:
Reaction 1: CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
This reaction is very slow in the absence of light. However, Cl2(g) can absorb light to form Cl atoms:
Reaction 2: Cl2(g) + hv → 2 Cl(g)
Once the Cl atoms are generated, they can catalyze the reaction of CH4 and Cl2, according to the following proposed mechanism:
Reaction 3: CH4(g) + Cl(g) → CH3(g) + HCl(g)
Reaction 4: CH3(g) + Cl2(g) → CH3Cl(g) + Cl(g)
The enthalpy changes and activation energies for these two reactions are tabulated as follows:
Reaction ΔH° (kJ/mol) Ea (kJ/mol)
3 +4 17
4 -109 4
(b) By using the data tabulated here, sketch a quantitative energy profile for the catalyzed reaction represented by reactions 3 and 4.
