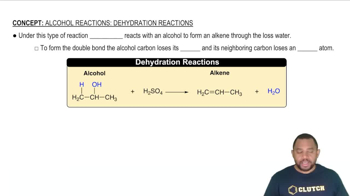
Consider the reaction of peroxydisulfate ion (S2O82-) with iodide ion (I-) in aqueous solution:
S2O82-(aq) + 3 I-(aq) → 2 SO42-(aq) + I3-(aq)
At a particular temperature, the initial rate of disappearance of S2O82- varies with reactant concentrations in the following manner:
Experiment [S2O82-] (M) [I-] (M) Initial Rate (M/s)
1 0.018 0.036 2.6 × 10-6
2 0.027 0.036 3.9 × 10-6
3 0.036 0.054 7.8 × 10-6
4 0.050 0.072 1.4 × 10-5
(a) Determine the rate law for the reaction and state the units of the rate constant.