(a) For the generic reaction A → B what quantity, when graphed versus time, will yield a straight line for a first-order reaction?
Ch.14 - Chemical Kinetics

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 14, Problem 41a
(a) The gas-phase decomposition of SO2Cl2, SO2Cl2(g) → SO2(g) + Cl2(g), is first order in SO2Cl2. At 600 K the half-life for this process is 2.3 × 105 s. What is the rate constant at this temperature?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the problem. We are given that the reaction is first order and we are given the half-life. We need to find the rate constant (k).
Step 2: Recall the formula for the rate constant in a first order reaction. The formula is k = ln(2) / t1/2, where t1/2 is the half-life of the reaction.
Step 3: Substitute the given half-life into the formula. The half-life is given as 2.3 * 10^5 s.
Step 4: Calculate the rate constant. Remember that ln(2) is approximately 0.693.
Step 5: The rate constant k is then the result of the calculation from step 4. This is the final answer.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
First-Order Reactions
First-order reactions are chemical reactions where the rate is directly proportional to the concentration of one reactant. This means that as the concentration of the reactant decreases, the rate of the reaction also decreases. The rate law for a first-order reaction can be expressed as rate = k[A], where k is the rate constant and [A] is the concentration of the reactant.
Recommended video:
Guided course

First-Order Reactions
Half-Life of a Reaction
The half-life of a reaction is the time required for the concentration of a reactant to decrease to half of its initial value. For first-order reactions, the half-life is constant and independent of the initial concentration, calculated using the formula t1/2 = 0.693/k, where k is the rate constant. This relationship is crucial for determining the rate constant from the given half-life.
Recommended video:
Guided course
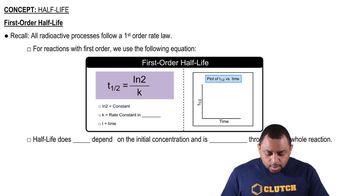
First-Order Half-Life
Rate Constant (k)
The rate constant (k) is a proportionality factor in the rate law that quantifies the speed of a reaction at a given temperature. It is specific to each reaction and varies with temperature. For first-order reactions, the rate constant can be derived from the half-life using the equation k = 0.693/t1/2, allowing us to calculate k when the half-life is known.
Recommended video:
Guided course

Equilibrium Constant K
Related Practice
Textbook Question
Textbook Question
(b) How can you calculate the rate constant for a first-order reaction from the graph you made in part (a)?
Textbook Question
The decomposition of sodium bicarbonate (baking soda), NaHCO3(s), into Na2CO3(s), H2O(l), and CO2(g) at constant pressure requires the addition of 85 kJ of heat per two moles of NaHCO3. (b) Draw an enthalpy diagram for the reaction.
Textbook Question
(b) At 320°C the rate constant is 2.2 × 10-5 s-1. What is the half-life at this temperature?
Textbook Question
As described in Exercise 14.41, the decomposition of sulfuryl chloride (SO2Cl2) is a first-order process. The rate constant for the decomposition at 660 K is 4.5 × 10-2 s-1. (a) If we begin with an initial SO2Cl2 pressure of 450 torr, what is the partial pressure of this substance after 60 s?
