Consider the following energy profile.
(b) How many intermediates are formed in the reaction?

 Verified step by step guidance
Verified step by step guidance


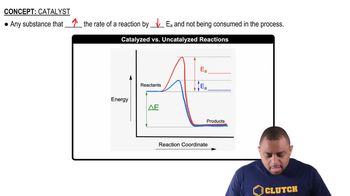
Consider the following energy profile.
(b) How many intermediates are formed in the reaction?
Consider the following energy profile.
(c) Which step is rate limiting?
The decomposition of hydrogen peroxide is catalyzed by iodide ion. The catalyzed reaction is thought to proceed by a two-step mechanism:
H2O2(aq) + I-(aq) → H2O(l) + IO-(aq) (slow)
IO-(aq) + H2O2(aq) → H2O(l) + O2(g) + I-(aq) (fast)
(b) Identify the intermediate, if any, in the mechanism.
The decomposition of hydrogen peroxide is catalyzed by iodide ion. The catalyzed reaction is thought to proceed by a two-step mechanism:
H2O2(aq) + I-(aq) → H2O(l) + IO-(aq) (slow)
IO-(aq) + H2O2(aq) → H2O(l) + O2(g) + I-(aq) (fast)
(c) Assuming that the first step of the mechanism is rate determining, predict the rate law for the overall process.
The reaction 2 NO1g2 + Cl21g2¡2 NOCl1g2 was performed and the following data were obtained under conditions of constant 3Cl24:
(a) Is the following mechanism consistent with the data? NO1g2 + Cl21g2ΔNOCl21g2 1fast2 NOCl21g2 + NO1g2¡2 NOCl1g2 1slow2