Textbook Question
(a) Based on the following reaction profile, how many intermediates are formed in the reaction A→D?

 Verified step by step guidance
Verified step by step guidance

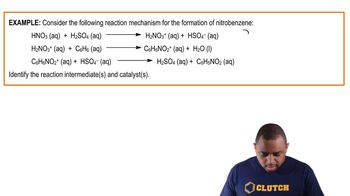
(a) Based on the following reaction profile, how many intermediates are formed in the reaction A→D?
(c) Which step is the fastest?
Consider the following energy profile.
(a) How many elementary reactions are in the reaction mechanism?
Consider the following energy profile.
(c) Which step is rate limiting?
The decomposition of hydrogen peroxide is catalyzed by iodide ion. The catalyzed reaction is thought to proceed by a two-step mechanism:
H2O2(aq) + I-(aq) → H2O(l) + IO-(aq) (slow)
IO-(aq) + H2O2(aq) → H2O(l) + O2(g) + I-(aq) (fast)
(a) Write the chemical equation for the overall process.