Based on the following reaction profile, how many intermediates are formed in the reaction A¡C? How many transition states are there? Which step, A¡B or B¡C, is the faster? For the reaction A¡C, is ΔE positive, negative, or zero? [Section 14.6]
Ch.14 - Chemical Kinetics

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 14, Problem 17
(c) Is the rate of disappearance of reactants always the same as the rate of appearance of products?
 Verified step by step guidance
Verified step by step guidance1
Understand the concept of reaction rates: The rate of disappearance of reactants and the rate of appearance of products are related to the stoichiometry of the chemical reaction.
Consider a general chemical reaction: \( aA + bB \rightarrow cC + dD \). Here, \( a, b, c, \) and \( d \) are the stoichiometric coefficients.
Express the rate of disappearance of reactants: The rate of disappearance of reactant \( A \) can be expressed as \( -\frac{1}{a} \frac{d[A]}{dt} \), and for \( B \) as \( -\frac{1}{b} \frac{d[B]}{dt} \).
Express the rate of appearance of products: The rate of appearance of product \( C \) can be expressed as \( \frac{1}{c} \frac{d[C]}{dt} \), and for \( D \) as \( \frac{1}{d} \frac{d[D]}{dt} \).
Compare the rates: The rates of disappearance and appearance are related through the stoichiometric coefficients. They are not necessarily equal unless the stoichiometric coefficients are the same for reactants and products.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Rates
Reaction rates refer to the speed at which reactants are converted into products in a chemical reaction. They can be expressed in terms of the change in concentration of reactants or products over time. Understanding reaction rates is crucial for analyzing how quickly a reaction proceeds and how it can be influenced by various factors such as temperature, concentration, and catalysts.
Recommended video:
Guided course

Average Rate of Reaction
Stoichiometry
Stoichiometry is the study of the quantitative relationships between the amounts of reactants and products in a chemical reaction. It is based on the balanced chemical equation, which indicates the ratio in which substances react and are produced. This concept is essential for determining how the rates of disappearance of reactants and appearance of products relate to each other in a given reaction.
Recommended video:
Guided course

Stoichiometry Concept
Conservation of Mass
The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction. This principle implies that the total mass of reactants must equal the total mass of products. Consequently, the rate of disappearance of reactants and the rate of appearance of products are related through their stoichiometric coefficients, ensuring that they are proportional in a balanced reaction.
Recommended video:
Guided course
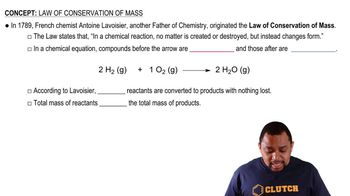
Law of Conservation of Mass
Related Practice
Textbook Question
Textbook Question
Consider the diagram that follows, which represents two steps in an overall reaction. The red spheres are oxygen, the blue ones nitrogen, and the green ones fluorine. (d) Write the rate law for the overall reaction if the first step is the slow, rate-determining step. [Section 14.6]
Textbook Question
(b) Name three factors that can affect the rate of a chemical reaction.
Textbook Question
(a) What are the units usually used to express the rates of reactions occurring in solution?
Textbook Question
(b) As the temperature increases, does the reaction rate increase or decrease?
