Textbook Question
Calculate the number of moles of solute present in each of the following aqueous solutions: (a) 600 mL of 0.250 M SrBr2,

 Verified step by step guidance
Verified step by step guidance

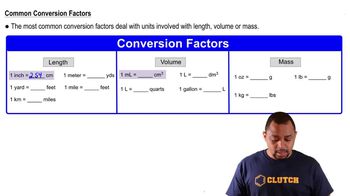

Calculate the number of moles of solute present in each of the following aqueous solutions: (a) 600 mL of 0.250 M SrBr2,
Calculate the number of moles of solute present in each of the following aqueous solutions: (b) 86.4 g of 0.180 m KCl,
Calculate the number of moles of solute present in each of the following aqueous solutions: (c) 124.0 g of a solution that is 6.45% glucose (C6H12O6) by mass.
Describe how you would prepare each of the following aqueous solutions, starting with solid KBr: (b) 125 g of 0.180 m KBr,
Describe how you would prepare each of the following aqueous solutions, starting with solid KBr: (c) 1.85 L of a solution that is 12.0% KBr by mass (the density of the solution is 1.10 g/mL)