A dilute aqueous solution of an organic compound soluble in water is formed by dissolving 2.35 g of the compound in water to form 0.250 L of solution. The resulting solution has an osmotic pressure of 0.605 atm at 25 °C. Assuming that the organic compound is a nonelectrolyte, what is its molar mass?

An “emulsifying agent” is a compound that helps stabilize a hydrophobic colloid in a hydrophilic solvent (or a hydrophilic colloid in a hydrophobic solvent). Which of the following choices is the best emulsifying agent? (a) CH3COOH, (b) CH3CH2CH2COOH, (c) CH3(CH2)11COOH, (d) CH3(CH2)11COONa.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Emulsifying Agents
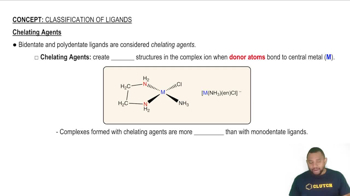
Hydrophilic and Hydrophobic Properties
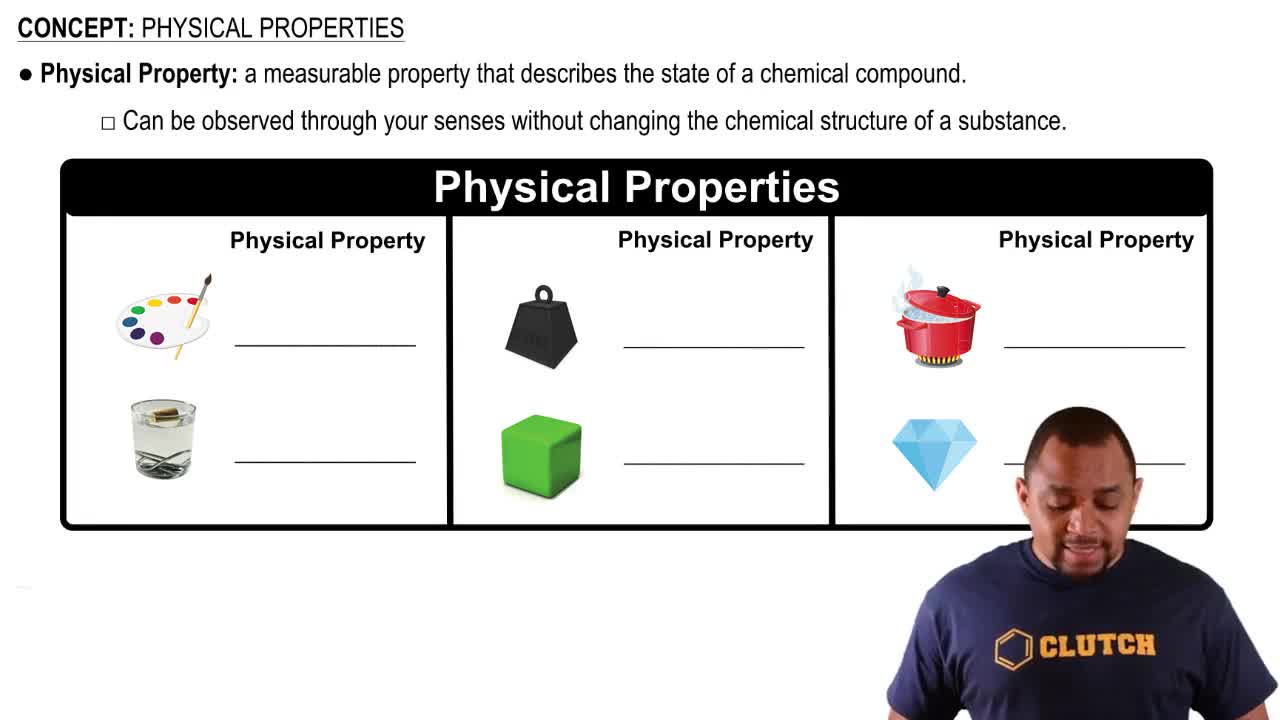
Colloids and Stability

The osmotic pressure of a 0.010 M aqueous solution of CaCl2 is found to be 0.674 atm at 25 °C. Calculate the van't Hoff factor, i, for the solution.
Aerosols are important components of the atmosphere. Does the presence of aerosols in the atmosphere increase or decrease the amount of sunlight that arrives at the Earth's surface, compared to an 'aerosol-free' atmosphere? Explain your reasoning.
The 'free-base' form of cocaine (C17H21NO4) and its protonated hydrochloride form (C17H22ClNO4) are shown below; the free-base form can be converted to the hydrochloride form with one equivalent of HCl. For clarity, not all the carbon and hydrogen atoms are shown; each vertex represents a carbon atom with the appropriate number of hydrogen atoms so that each carbon makes four bonds to other atoms
(a) One of these forms of cocaine is relatively water-soluble: which form, the free base or the hydrochloride?
