An increase in temperature causes most metals to undergo thermal expansion, which means the volume of the metal increases upon heating. How does thermal expansion affect the unit cell length? What is the effect of an increase in temperature on the density of a metal?

The molecular-orbital diagrams for two- and four-atom linear chains of lithium atoms are shown in Figure 12.22. Construct a molecular-orbital diagram for a chain containing six lithium atoms and use it to answer the following questions: (a) How many molecular orbitals are there in the diagram?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Molecular Orbitals
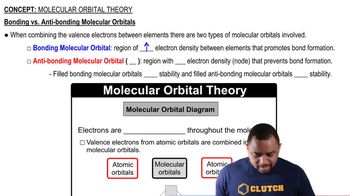
Atomic Orbitals in Lithium
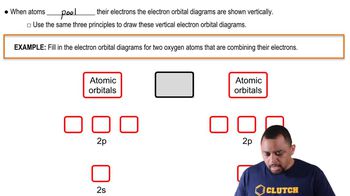
Counting Molecular Orbitals

State whether each sentence is true or false: (a) Metals have high electrical conductivities because the electrons in the metal are delocalized. (c) Metals have large thermal conductivities because they expand when heated. (d) Metals have small thermal conductivities because the delocalized electrons cannot easily transfer the kinetic energy imparted to the metal from heat.
State whether each sentence is true or false: (b) Metals have high electrical conductivities because they are denser than other solids.
The molecular-orbital diagrams for two- and four-atom linear chains of lithium atoms are shown in Figure 12.22. Construct a molecular-orbital diagram for a chain containing six lithium atoms and use it to answer the following questions: (c) How many nodes are in the highest-energy molecular orbital?
The molecular-orbital diagrams for two- and four-atom linear chains of lithium atoms are shown in Figure 12.22. Construct a molecular-orbital diagram for a chain containing six lithium atoms and use it to answer the following questions: (e) How many nodes are in the lowest-energy unoccupied molecular orbital (LUMO)?
Repeat Exercise 12.51 for a linear chain of eight lithium atoms. (b) How many nodes are in the lowest-energy molecular orbital? (c) How many nodes are in the highestenergy molecular orbital? (d) How many nodes are in the highest-energy occupied molecular orbital (HOMO)? (e) How many nodes are in the lowest-energy unoccupied molecular orbital (LUMO)?
