Textbook Question
(a) What is a monomer? (b) Which of these molecules can be used as a monomer: ethanol, ethene (also called ethylene), methane?

 Verified step by step guidance
Verified step by step guidance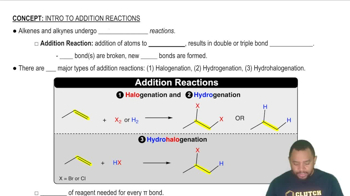
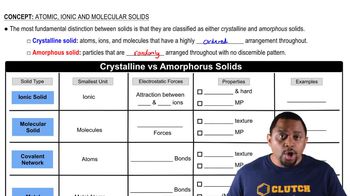

(a) What is a monomer? (b) Which of these molecules can be used as a monomer: ethanol, ethene (also called ethylene), methane?
Write a balanced chemical equation for the formation of a polymer via a condensation reaction from the monomers succinic acid 1HOOCCH2CH2COOH2 and ethylenediamine 1H2NCH2CH2NH22.